Cell ( IF 45.5 ) Pub Date : 2021-08-06 , DOI: 10.1016/j.cell.2021.07.026 Justin Taft 1 , Michael Markson 1 , Diana Legarda 2 , Roosheel Patel 1 , Mark Chan 3 , Louise Malle 1 , Ashley Richardson 1 , Conor Gruber 1 , Marta Martín-Fernández 1 , Grazia M S Mancini 4 , Jan A M van Laar 5 , Philomine van Pelt 6 , Sofija Buta 1 , Beatrijs H A Wokke 7 , Ira K D Sabli 8 , Vanessa Sancho-Shimizu 8 , Pallavi Pimpale Chavan 9 , Oskar Schnappauf 10 , Raju Khubchandani 11 , Müşerref Kasap Cüceoğlu 12 , Seza Özen 12 , Daniel L Kastner 10 , Adrian T Ting 3 , Ivona Aksentijevich 10 , Iris H I M Hollink 4 , Dusan Bogunovic 1
|
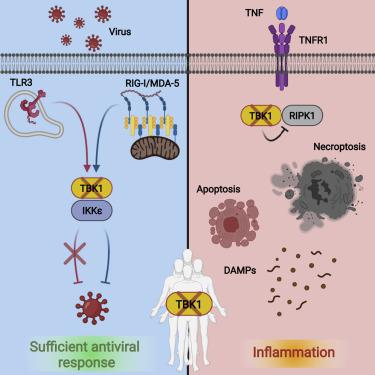
TANK binding kinase 1 (TBK1) regulates IFN-I, NF-κB, and TNF-induced RIPK1-dependent cell death (RCD). In mice, biallelic loss of TBK1 is embryonically lethal. We discovered four humans, ages 32, 26, 7, and 8 from three unrelated consanguineous families with homozygous loss-of-function mutations in TBK1. All four patients suffer from chronic and systemic autoinflammation, but not severe viral infections. We demonstrate that TBK1 loss results in hypomorphic but sufficient IFN-I induction via RIG-I/MDA5, while the system retains near intact IL-6 induction through NF-κB. Autoinflammation is driven by TNF-induced RCD as patient-derived fibroblasts experienced higher rates of necroptosis in vitro, and CC3 was elevated in peripheral blood ex vivo. Treatment with anti-TNF dampened the baseline circulating inflammatory profile and ameliorated the clinical condition in vivo. These findings highlight the plasticity of the IFN-I response and underscore a cardinal role for TBK1 in the regulation of RCD.
中文翻译:

人类 TBK1 缺乏导致由 TNF 诱导的细胞死亡驱动的自身炎症
TANK 结合激酶 1 (TBK1) 调节 IFN-I、NF-κB 和 TNF 诱导的 RIPK1 依赖性细胞死亡 (RCD)。在小鼠中,TBK1 的双等位基因丢失在胚胎上是致命的。我们从三个不相关的近亲家族中发现了四个人,年龄分别为 32、26、7 和 8 岁,在TBK1中具有纯合功能丧失突变。所有四名患者都患有慢性和全身性自身炎症,但没有严重的病毒感染。我们证明 TBK1 损失导致通过 RIG-I/MDA5 诱导低形态但足够的 IFN-I,而系统通过 NF-κB 保留接近完整的 IL-6 诱导。自身炎症由 TNF 诱导的 RCD 驱动,因为患者来源的成纤维细胞在体外经历了更高的坏死性凋亡率,并且离体外周血中 CC3 升高. 用抗 TNF 治疗抑制了基线循环炎症谱并改善了体内临床状况。这些发现突出了 IFN-I 反应的可塑性,并强调了 TBK1 在 RCD 调节中的主要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号