当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Enantioselective [8+4] Cycloadditions of Isobenzofulvenes for the Construction of Bicyclo[4.2.1]nonanes
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-08-06 , DOI: 10.1002/cjoc.202100250 Jianhong Zhao 1 , Xing Zheng 1 , Yan‐Shan Gao 1 , Jia Mao 1 , Shu‐Xiao Wu 1 , Wu‐Lin Yang 1 , Xiaoyan Luo 1 , Wei‐Ping Deng 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-08-06 , DOI: 10.1002/cjoc.202100250 Jianhong Zhao 1 , Xing Zheng 1 , Yan‐Shan Gao 1 , Jia Mao 1 , Shu‐Xiao Wu 1 , Wu‐Lin Yang 1 , Xiaoyan Luo 1 , Wei‐Ping Deng 1
Affiliation
|
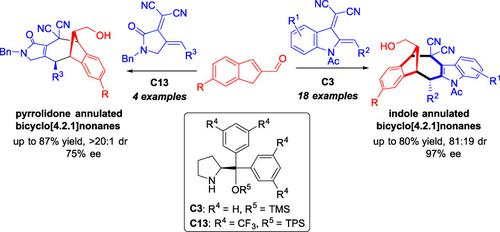
The [8+4] cycloaddition of indene-2-carbaldehydes with indole-2,3-quinodimethanes and pyrrolidone-3,4-dienes is described, affording indole and pyrrolidone annulated bicyclo[4.2.1]nonanes in a highly peri-, diastereo-, and enantioselective fashion in the presence of a secondary amine catalyst. This reaction, which proceeds through catalytically generated isobenzofulvenes, represents the first asymmetric version of high-order [8+4] cycloaddition.
中文翻译:

用于构建双环[4.2.1]壬烷的异苯并富烯的有机催化对映选择性[8+4]环加成
描述了茚2-甲醛与indole-2,3-quinodimethanes 和pyrrolidone-3,4-dienes 的[8+4] 环加成,在高度环-,在仲胺催化剂存在下的非对映选择性和对映选择性方式。该反应通过催化生成的异苯并富烯进行,代表了高阶 [8+4] 环加成的第一个不对称形式。
更新日期:2021-08-06

中文翻译:

用于构建双环[4.2.1]壬烷的异苯并富烯的有机催化对映选择性[8+4]环加成
描述了茚2-甲醛与indole-2,3-quinodimethanes 和pyrrolidone-3,4-dienes 的[8+4] 环加成,在高度环-,在仲胺催化剂存在下的非对映选择性和对映选择性方式。该反应通过催化生成的异苯并富烯进行,代表了高阶 [8+4] 环加成的第一个不对称形式。












































 京公网安备 11010802027423号
京公网安备 11010802027423号