当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Permanganate Reduction by Hydrogen Peroxide: Formation of Reactive Manganese Species and Superoxide and Enhanced Micropollutant Abatement
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-08-05 , DOI: 10.1021/acsestengg.1c00138 Ke Xu 1 , Urs von Gunten 1, 2, 3
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-08-05 , DOI: 10.1021/acsestengg.1c00138 Ke Xu 1 , Urs von Gunten 1, 2, 3
Affiliation
|
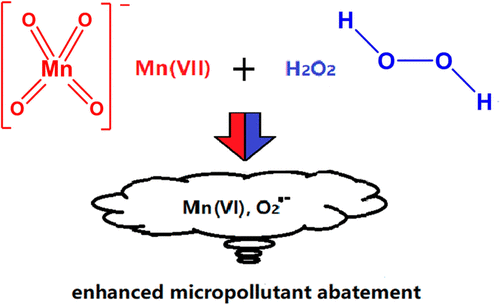
Permanganate (MnO4–, Mn(VII)) is widely applied at the initial stage of water treatment to, e.g., abate taste and odor compounds, Mn(II) and Fe(II). However, its selectivity limits its application for micropollutant abatement. Hydrogen peroxide (H2O2), which is commonly applied in advanced oxidation processes, was found to quickly react with Mn(VII) in the pH-range 6.0–8.5. A lag-phase was followed by a rapid reduction of Mn(VII) to Mn(VI), with a proposed catalysis by the deprotonated form of Mn(VI), for the electron transfer of the [H2O2–OMnO3]− complex. At molar [H2O2]0:[Mn(VII)]0 ratios ≤ 1 and pH 7.5, a one-electron reduction of Mn(VII) by H2O2 potentially formed Mn(VI) and superoxide radical (O2•–), with O2•– yields approaching 100% based on the consumed Mn(VII). Mn(VII)–H2O2 significantly enhanced the abatement of the Mn(VII)-resistant compound ciprofloxacin and its transformation products, and quenching of Mn(VI) by Ba2+ completely inhibited this enhancement effect. At molar [H2O2]0:[Mn(VII)]0 ratios of 2–10, O2•– yields of 145–245% indicate that other H2O2-reactive manganese species might also be formed. Among them, Mn(II) was found to produce O2•– by reaction with H2O2 at a relatively low rate with overstoichiometric yields. Overall, this study provides evidence that a combination of Mn(VII) with H2O2 might be a novel option for enhanced micropollutant abatement in water treatment.
中文翻译:

过氧化氢还原高锰酸盐:反应性锰物种和超氧化物的形成和增强的微污染物减排
高锰酸盐 (MnO 4 – , Mn(VII)) 广泛应用于水处理的初始阶段,例如减少味道和气味的化合物,Mn(II) 和 Fe(II)。然而,其选择性限制了其在微污染物减排中的应用。发现通常用于高级氧化工艺的过氧化氢 (H 2 O 2 ) 在 6.0-8.5 的 pH 范围内与 Mn(VII) 快速反应。滞后阶段之后是 Mn(VII) 快速还原为 Mn(VI),建议通过去质子化形式的 Mn(VI) 催化 [H 2 O 2 –OMnO 3 ]的电子转移-复杂。摩尔数 [H 2 O 2 ] 0:[Mn(VII)] 0比率 ≤ 1 和 pH 7.5,Mn(VII) 被 H 2 O 2的单电子还原可能形成的 Mn(VI) 和超氧自由基 (O 2 •– ),与 O 2 • –基于消耗的 Mn(VII) 产量接近 100%。Mn(VII)–H 2 O 2显着增强了耐Mn(VII) 化合物环丙沙星及其转化产物的减弱,Ba 2+对Mn(VI) 的淬火完全抑制了这种增强作用。在 [H 2 O 2 ] 0 :[Mn(VII)] 0摩尔比为 2–10 时,O 2 •–145-245% 的产量表明也可能形成其他 H 2 O 2反应性锰物种。其中,Mn(II) 被发现通过与 H 2 O 2以相对低的速率反应生成 O 2 •–并产生超化学计量的产率。总体而言,这项研究提供的证据表明,Mn(VII) 与 H 2 O 2 的组合可能是增强水处理中微污染物减排的新选择。
更新日期:2021-10-08
中文翻译:

过氧化氢还原高锰酸盐:反应性锰物种和超氧化物的形成和增强的微污染物减排
高锰酸盐 (MnO 4 – , Mn(VII)) 广泛应用于水处理的初始阶段,例如减少味道和气味的化合物,Mn(II) 和 Fe(II)。然而,其选择性限制了其在微污染物减排中的应用。发现通常用于高级氧化工艺的过氧化氢 (H 2 O 2 ) 在 6.0-8.5 的 pH 范围内与 Mn(VII) 快速反应。滞后阶段之后是 Mn(VII) 快速还原为 Mn(VI),建议通过去质子化形式的 Mn(VI) 催化 [H 2 O 2 –OMnO 3 ]的电子转移-复杂。摩尔数 [H 2 O 2 ] 0:[Mn(VII)] 0比率 ≤ 1 和 pH 7.5,Mn(VII) 被 H 2 O 2的单电子还原可能形成的 Mn(VI) 和超氧自由基 (O 2 •– ),与 O 2 • –基于消耗的 Mn(VII) 产量接近 100%。Mn(VII)–H 2 O 2显着增强了耐Mn(VII) 化合物环丙沙星及其转化产物的减弱,Ba 2+对Mn(VI) 的淬火完全抑制了这种增强作用。在 [H 2 O 2 ] 0 :[Mn(VII)] 0摩尔比为 2–10 时,O 2 •–145-245% 的产量表明也可能形成其他 H 2 O 2反应性锰物种。其中,Mn(II) 被发现通过与 H 2 O 2以相对低的速率反应生成 O 2 •–并产生超化学计量的产率。总体而言,这项研究提供的证据表明,Mn(VII) 与 H 2 O 2 的组合可能是增强水处理中微污染物减排的新选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号