当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for the Interaction of Fibrin with the Very Low-Density Lipoprotein Receptor Revealed by NMR and Site-Directed Mutagenesis
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-05 , DOI: 10.1021/acs.biochem.1c00378 James M Gruschus 1 , Sergiy Yakovlev 2 , Koyeli Banerjee 1 , Leonid Medved 2 , Nico Tjandra 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-05 , DOI: 10.1021/acs.biochem.1c00378 James M Gruschus 1 , Sergiy Yakovlev 2 , Koyeli Banerjee 1 , Leonid Medved 2 , Nico Tjandra 1
Affiliation
|
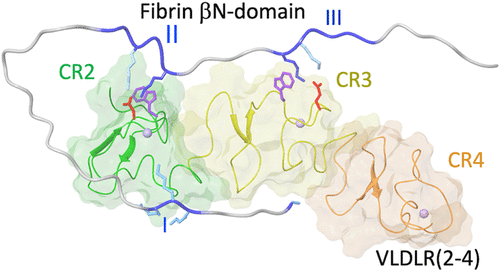
Interaction of fibrin with the very low-density lipoprotein receptor (VLDLR) promotes transendothelial migration of leukocytes and thereby inflammation. To establish the structural basis for this interaction, we have previously localized the VLDLR-binding site to fibrin βN-domains including fibrin β chain sequence 15–64 and determined the NMR solution structure of the VLDLR(2–4) fragment containing fibrin-binding CR domains 2–4 of VLDLR. In this study, we identified amino acid residues in VLDLR and the βN-domains that are involved in the interaction using NMR and site-directed mutagenesis. The results obtained revealed that Lys47 and Lys53 of the second and third positively charged clusters of the βN-domain, respectively, interact with Trp20 and Asp25 of the CR2 domain and Trp63 and Glu68 of the CR3 domain, respectively. This finding indicates that Lys residues of the βN-domain interact with the Lys-binding site of the CR domains in a manner proposed earlier for the interaction of other members of the LDL receptor family with their ligands. In addition, Gly15 of the βN-domain and its first positively charged cluster contribute to the high-affinity interaction with VLDLR. Molecular modeling based on the results obtained and analysis of the previously published structures of such domains complexed with RAP and HRV2 allowed us to propose a model of interaction of fibrin βN-domains with the fibrin-binding CR domains of the VLDL receptor.
中文翻译:

核磁共振和定点诱变揭示纤维蛋白与极低密度脂蛋白受体相互作用的结构基础
纤维蛋白与极低密度脂蛋白受体(VLDLR)的相互作用促进白细胞跨内皮迁移,从而促进炎症。为了建立这种相互作用的结构基础,我们之前将 VLDLR 结合位点定位于纤维蛋白 βN 结构域,包括纤维蛋白 β 链序列 15–64,并确定了包含纤维蛋白结合的 VLDLR(2–4) 片段的 NMR 溶液结构VLDLR 的 CR 结构域 2-4。在这项研究中,我们使用 NMR 和定点诱变鉴定了 VLDLR 中参与相互作用的氨基酸残基和 βN 结构域。获得的结果表明,βN结构域的第二和第三带正电簇的Lys47和Lys53分别与CR2结构域的Trp20和Asp25以及CR3结构域的Trp63和Glu68相互作用。这一发现表明,βN 结构域的 Lys 残基与 CR 结构域的 Lys 结合位点以先前提出的 LDL 受体家族其他成员与其配体相互作用的方式相互作用。此外,βN 结构域的 Gly15 及其第一个带正电荷的簇有助于与 VLDLR 的高亲和力相互作用。基于所获得的结果的分子建模以及对先前发表的与 RAP 和 HRV2 复合的此类结构域的结构的分析使我们能够提出纤维蛋白 βN 结构域与 VLDL 受体的纤维蛋白结合 CR 结构域相互作用的模型。
更新日期:2021-08-24
中文翻译:

核磁共振和定点诱变揭示纤维蛋白与极低密度脂蛋白受体相互作用的结构基础
纤维蛋白与极低密度脂蛋白受体(VLDLR)的相互作用促进白细胞跨内皮迁移,从而促进炎症。为了建立这种相互作用的结构基础,我们之前将 VLDLR 结合位点定位于纤维蛋白 βN 结构域,包括纤维蛋白 β 链序列 15–64,并确定了包含纤维蛋白结合的 VLDLR(2–4) 片段的 NMR 溶液结构VLDLR 的 CR 结构域 2-4。在这项研究中,我们使用 NMR 和定点诱变鉴定了 VLDLR 中参与相互作用的氨基酸残基和 βN 结构域。获得的结果表明,βN结构域的第二和第三带正电簇的Lys47和Lys53分别与CR2结构域的Trp20和Asp25以及CR3结构域的Trp63和Glu68相互作用。这一发现表明,βN 结构域的 Lys 残基与 CR 结构域的 Lys 结合位点以先前提出的 LDL 受体家族其他成员与其配体相互作用的方式相互作用。此外,βN 结构域的 Gly15 及其第一个带正电荷的簇有助于与 VLDLR 的高亲和力相互作用。基于所获得的结果的分子建模以及对先前发表的与 RAP 和 HRV2 复合的此类结构域的结构的分析使我们能够提出纤维蛋白 βN 结构域与 VLDL 受体的纤维蛋白结合 CR 结构域相互作用的模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号