当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Acyclic Orthogonally Functionalized Compounds Bearing a Quaternary Stereocenter Using Chiral Ammonium Salt Catalysis
ChemistryOpen ( IF 2.5 ) Pub Date : 2021-08-05 , DOI: 10.1002/open.202100162 Katharina Röser 1 , Bettina Berger 2 , Michael Widhalm 2 , Mario Waser 1
ChemistryOpen ( IF 2.5 ) Pub Date : 2021-08-05 , DOI: 10.1002/open.202100162 Katharina Röser 1 , Bettina Berger 2 , Michael Widhalm 2 , Mario Waser 1
Affiliation
|
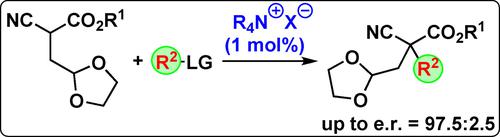
We herein report an asymmetric protocol to access a series of orthogonally functionalized acyclic chiral target molecules containing a quaternary stereogenic center by carrying out the enantioselective α-alkylation of novel orthogonally functionalized dioxolane-containing cyanoacetates under chiral ammonium salt catalysis. By using just 1 mol % of Maruoka's spirocyclic ammonium salt catalysts enantioselectivities up to e.r.=97.5 : 2.5 could be achieved and further functional group manipulations of the products were carried out as well.
中文翻译:

手性铵盐催化对映选择性合成带有季立构中心的无环正交官能化化合物
我们在此报告了一种不对称方案,通过在手性铵盐催化下对新型正交功能化的含二氧戊环的氰基乙酸酯进行对映选择性α-烷基化,获得一系列含有季立体中心的正交功能化的无环手性目标分子。仅使用 1 mol% 的 Maruoka 螺环铵盐催化剂即可实现高达 er=97.5 : 2.5 的对映选择性,并且还可以对产物进行进一步的官能团操作。
更新日期:2021-08-05
中文翻译:

手性铵盐催化对映选择性合成带有季立构中心的无环正交官能化化合物
我们在此报告了一种不对称方案,通过在手性铵盐催化下对新型正交功能化的含二氧戊环的氰基乙酸酯进行对映选择性α-烷基化,获得一系列含有季立体中心的正交功能化的无环手性目标分子。仅使用 1 mol% 的 Maruoka 螺环铵盐催化剂即可实现高达 er=97.5 : 2.5 的对映选择性,并且还可以对产物进行进一步的官能团操作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号