当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superionic Fluorinated Halide Solid Electrolytes for Highly Stable Li-Metal in All-Solid-State Li Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-08-04 , DOI: 10.1002/aenm.202101915 Tianwei Yu 1, 2, 3 , Jianwen Liang 4 , Liang Luo 5, 6 , Limin Wang 3, 7 , Feipeng Zhao 4 , Guofeng Xu 1, 2, 3 , Xiangtao Bai 1, 2, 3 , Rong Yang 1, 2, 3 , Shangqian Zhao 1, 2, 3 , Jiantao Wang 1, 2, 3 , Jinqiu Yu 5, 6 , Xueliang Sun 4
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-08-04 , DOI: 10.1002/aenm.202101915 Tianwei Yu 1, 2, 3 , Jianwen Liang 4 , Liang Luo 5, 6 , Limin Wang 3, 7 , Feipeng Zhao 4 , Guofeng Xu 1, 2, 3 , Xiangtao Bai 1, 2, 3 , Rong Yang 1, 2, 3 , Shangqian Zhao 1, 2, 3 , Jiantao Wang 1, 2, 3 , Jinqiu Yu 5, 6 , Xueliang Sun 4
Affiliation
|
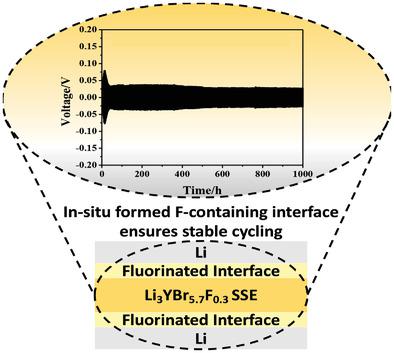
The halide solid-state electrolytes (SSEs) have received significant attention due to their high ionic conductivity and desirable compatibility with cathode materials. However, the reduction potential of the halide is still >0.6 V (versus Li/Li+). Reduction stability is still one of the challenges that need to be addressed. The fluorides have a wide electrochemical stability window due to the large electronegativity of F–. In contrast, Li3YBr6 (LYB) bromides have a narrower electrochemical window, although they have high lithium ion conductivity (>10–3 S cm–1). Herein, a fluorine doping strategy is employed. The interfacial stability between fluoride-doped bromides and lithium metal is researched by cycling of lithium symmetric cells. Li plating/stripping can maintain over 1000 h at 0.75 mA cm–2. Interfacial protection mechanisms investigated by X-ray photoelectron spectroscopy. A fluoride-rich interfacial layer is formed in situ during the cycle, which achieves inhibition of the reduction. The Li metal treated fluorine doping of LYB exhibits significant potential in full cells. In fact, the induction of a stable in situ interfacial layer by fluorine doping can effectively improve the interfacial stability of bromides to lithium metal. Fluorine-doped modification offers a new attempt to realize lithium metal applications in all-solid-state lithium batteries.
中文翻译:

用于全固态锂电池中高稳定性锂金属的超离子氟化卤化物固体电解质
卤化物固态电解质(SSE)由于其高离子电导率和与正极材料的理想相容性而受到了极大的关注。然而,卤化物的还原电位仍然 >0.6 V(相对于 Li/Li +)。还原稳定性仍然是需要解决的挑战之一。由于 F -的大电负性,氟化物具有宽的电化学稳定性窗口。相比之下,Li 3 YBr 6 (LYB) 溴化物虽然具有较高的锂离子电导率 (>10 –3 S cm –1)。在此,采用氟掺杂策略。通过锂对称电池的循环研究了掺氟溴化物和锂金属之间的界面稳定性。锂电镀/剥离可以在 0.75 mA cm –2 下保持超过 1000 小时。通过 X 射线光电子能谱研究的界面保护机制。在循环过程中原位形成富含氟化物的界面层,从而实现对还原的抑制。LYB 的锂金属处理的氟掺杂在全电池中表现出巨大的潜力。事实上,通过氟掺杂诱导稳定的原位界面层可以有效提高溴化物与金属锂的界面稳定性。掺氟改性为实现锂金属在全固态锂电池中的应用提供了新的尝试。
更新日期:2021-09-23
中文翻译:

用于全固态锂电池中高稳定性锂金属的超离子氟化卤化物固体电解质
卤化物固态电解质(SSE)由于其高离子电导率和与正极材料的理想相容性而受到了极大的关注。然而,卤化物的还原电位仍然 >0.6 V(相对于 Li/Li +)。还原稳定性仍然是需要解决的挑战之一。由于 F -的大电负性,氟化物具有宽的电化学稳定性窗口。相比之下,Li 3 YBr 6 (LYB) 溴化物虽然具有较高的锂离子电导率 (>10 –3 S cm –1)。在此,采用氟掺杂策略。通过锂对称电池的循环研究了掺氟溴化物和锂金属之间的界面稳定性。锂电镀/剥离可以在 0.75 mA cm –2 下保持超过 1000 小时。通过 X 射线光电子能谱研究的界面保护机制。在循环过程中原位形成富含氟化物的界面层,从而实现对还原的抑制。LYB 的锂金属处理的氟掺杂在全电池中表现出巨大的潜力。事实上,通过氟掺杂诱导稳定的原位界面层可以有效提高溴化物与金属锂的界面稳定性。掺氟改性为实现锂金属在全固态锂电池中的应用提供了新的尝试。









































 京公网安备 11010802027423号
京公网安备 11010802027423号