Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-08-05 , DOI: 10.1016/j.bmc.2021.116349 Wan-Chen Tsai 1 , Nathan C Gilbert 2 , Amanda Ohler 3 , Michelle Armstrong 1 , Steven Perry 1 , Chakrapani Kalyanaraman 4 , Adam Yasgar 5 , Ganesha Rai 5 , Anton Simeonov 5 , Ajit Jadhav 5 , Melissa Standley 1 , Hsiau-Wei Lee 1 , Phillip Crews 1 , Anthony T Iavarone 6 , Matthew P Jacobson 4 , David B Neau 7 , Adam R Offenbacher 3 , Marcia Newcomer 2 , Theodore R Holman 1
|
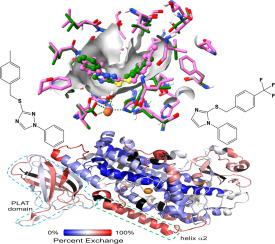
Human epithelial 15-lipoxygenase-2 (h15-LOX-2, ALOX15B) is expressed in many tissues and has been implicated in atherosclerosis, cystic fibrosis and ferroptosis. However, there are few reported potent/selective inhibitors that are active ex vivo. In the current work, we report newly discovered molecules that are more potent and structurally distinct from our previous inhibitors, MLS000545091 and MLS000536924 (Jameson et al, PLoS One, 2014, 9, e104094), in that they contain a central imidazole ring, which is substituted at the 1-position with a phenyl moiety and with a benzylthio moiety at the 2-position. The initial three molecules were mixed-type, non-reductive inhibitors, with IC50 values of 0.34 ± 0.05 μM for MLS000327069, 0.53 ± 0.04 μM for MLS000327186 and 0.87 ± 0.06 μM for MLS000327206 and greater than 50-fold selectivity versus h5-LOX, h12-LOX, h15-LOX-1, COX-1 and COX-2. A small set of focused analogs was synthesized to demonstrate the validity of the hits. In addition, a binding model was developed for the three imidazole inhibitors based on computational docking and a co-structure of h15-LOX-2 with MLS000536924. Hydrogen/deuterium exchange (HDX) results indicate a similar binding mode between MLS000536924 and MLS000327069, however, the latter restricts protein motion of helix-α2 more, consistent with its greater potency. Given these results, we designed, docked, and synthesized novel inhibitors of the imidazole scaffold and confirmed our binding mode hypothesis. Importantly, four of the five inhibitors mentioned above are active in an h15-LOX-2/HEK293 cell assay and thus they could be important tool compounds in gaining a better understanding of h15-LOX-2’s role in human biology. As such, a suite of similar pharmacophores that target h15-LOX-2 both in vitro and ex vivo are presented in the hope of developing them as therapeutic agents.
中文翻译:

人类上皮 15-脂氧合酶 2 新型抑制剂的动力学和结构研究
人上皮 15-脂氧合酶 2 (h15-LOX-2, ALOX15B) 在许多组织中表达,并与动脉粥样硬化、囊性纤维化和铁死亡有关。然而,很少有报道的有效/选择性抑制剂在体外具有活性。在目前的工作中,我们报告了新发现的分子,这些分子与我们之前的抑制剂MLS000545091和MLS000536924 (Jameson et al, PLoS One, 2014, 9, e104094)相比更有效且结构不同,因为它们含有一个中心咪唑环,其中在1-位被苯基部分取代并且在2-位被苄硫基部分取代。最初的三个分子是混合型非还原性抑制剂,IC 50值为 0.34 ± 0.05 μMMLS000327069,MLS000327186 为 0.53 ± 0.04 μM,MLS000327206为0.87 ± 0.06 μM,与 h5-LOX、h12-LOX、 h15 -LOX-1、COX-1 和 COX-2 相比,选择性高出 50 倍以上。合成了一小组集中的类似物来证明命中的有效性。此外,基于计算对接和 h15-LOX-2 与MLS000536924的共结构,开发了三种咪唑抑制剂的结合模型。氢/氘交换 (HDX) 结果表明MLS000536924和MLS000327069 之间的结合模式相似,然而,后者更多地限制了 helix-α2 的蛋白质运动,与其更强的效力一致。鉴于这些结果,我们设计、对接和合成了咪唑支架的新型抑制剂,并证实了我们的结合模式假设。重要的是,上述五种抑制剂中有四种在 h15-LOX-2/HEK293 细胞试验中具有活性,因此它们可能是更好地了解 h15-LOX-2 在人类生物学中的作用的重要工具化合物。因此,提出了一套在体外和离体靶向 h15-LOX-2 的类似药效团,希望将它们开发为治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号