Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-08-04 , DOI: 10.1016/j.bmc.2021.116347 Wan-Chen Tsai 1 , Ansari M Aleem 1 , Jennyfer Tena 1 , Mirella Rivera-Velazquez 1 , Harman Singh Brah 2 , Sarvind Tripathi 1 , Melinee D'silva 3 , Jerry L Nadler 3 , Chakrapani Kalyanaraman 2 , Matthew P Jacobson 2 , Theodore Holman 1
|
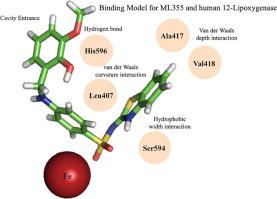
Human platelet 12-(S)-Lipoxygenase (12-LOX) is a fatty acid metabolizing oxygenase that plays an important role in platelet activation and cardiometabolic disease. ML355 is a specific 12-LOX inhibitor that has been shown to decrease thrombosis without prolonging hemostasis and protect human pancreatic islets from inflammatory injury. It has an amenable drug-like scaffold with nM potency and encouraging ADME and PK profiles, but its binding mode to the active site of 12-LOX remains unclear. In the current work, we combined computational modeling and experimental mutagenesis to propose a model in which ML355 conforms to the “U” shape of the 12-LOX active site, with the phenyl linker region wrapping around L407. The benzothiazole of ML355 extends into the bottom of the active site cavity, pointing towards residues A417 and V418. However, reducing the active site depth alone did not affect ML355 potency. In order to lower the potency of ML355, the cavity needed to be reduced in both length and width. In addition, H596 appears to position ML355 in the active site through an interaction with the p-methoxy catechol moiety of ML355. Combined, this binding model suggested that the benzothiazole of ML355 could be enlarged. Therefore, a napthyl-benzothiazole derivative of ML355, Lox12Slug001, was synthesized and shown to have 7.2-fold greater potency than ML355. This greater potency is proposed to be due to additional van der Waals interactions and pi-pi stacking with F414 and F352. Lox12Slug001 was also shown to be highly selective against 12-LOX relative to the other LOX isozymes and more importantly, it showed activity in rescuing human islets exposed to inflammatory cytokines with comparable potency to ML355. Further studies are currently being pursued to derivatize ML355 in order to optimize the additional space in the active site, while maintaining acceptable drug-like properties.
中文翻译:

对接和诱变研究改进了用于人血小板 12-脂氧合酶的 ML355 抑制剂的开发
人血小板 12-(S)-脂氧合酶 (12-LOX) 是一种脂肪酸代谢加氧酶,在血小板活化和心脏代谢疾病中起重要作用。ML355是一种特异性 12-LOX 抑制剂,已被证明可减少血栓形成而不延长止血时间,并保护人类胰岛免受炎症损伤。它有一个合适的药物样支架,具有 nM 效力和令人鼓舞的 ADME 和 PK 谱,但其与 12-LOX 活性位点的结合模式仍不清楚。在目前的工作中,我们结合计算建模和实验诱变提出了一个模型,其中ML355符合 12-LOX 活性位点的“U”形,苯基接头区域环绕 L407。ML355苯并噻唑延伸到活性位点腔的底部,指向残基 A417 和 V418。然而,仅降低活性位点深度并不影响ML355 的效力。为了降低ML355的效力,需要减小空腔的长度和宽度。此外,H596 似乎通过与 ML355 的对甲氧基儿茶酚部分的相互作用将 ML355 定位在活性位点。结合起来,这种结合模型表明ML355的苯并噻唑可以扩大。因此,合成了 ML355 的萘基苯并噻唑衍生物Lox12Slug001,其效力是ML355的 7.2 倍. 这种更大的效力被认为是由于额外的范德华相互作用以及与 F414 和 F352 的 pi-pi 堆叠。相对于其他 LOX 同工酶, Lox12Slug001还显示出对 12-LOX 的高度选择性,更重要的是,它在拯救暴露于炎性细胞因子的人类胰岛方面表现出活性,其效力与ML355相当。目前正在进行进一步的研究以衍生ML355,以优化活性位点的额外空间,同时保持可接受的药物样特性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号