Journal of Hepatology ( IF 26.8 ) Pub Date : 2021-08-05 , DOI: 10.1016/j.jhep.2021.07.033 Cornelius Engelmann 1 , Adam Herber 2 , Annegret Franke 3 , Tony Bruns 4 , Philipp Reuken 5 , Ingolf Schiefke 6 , Alexander Zipprich 7 , Stefan Zeuzem 8 , Tobias Goeser 9 , Ali Canbay 10 , Christoph Berg 11 , Jonel Trebicka 12 , Frank E Uschner 13 , Johannes Chang 14 , Tobias Mueller 15 , Niklas Aehling 2 , Moritz Schmelzle 16 , Katrin Splith 16 , Frank Lammert 17 , Christian M Lange 18 , Christoph Sarrazin 19 , Christian Trautwein 20 , Michael Manns 21 , Dieter Häussinger 22 , Jan Pfeiffenberger 23 , Peter R Galle 24 , Anett Schmiedeknecht 3 , Thomas Berg 2
|
Background & Aims
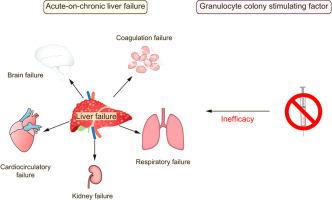
Based on positive results from small single center studies, granulocyte-colony stimulating factor (G-CSF) is being widely used for the treatment of patients with acute-on-chronic liver failure (ACLF). Herein, we aimed to evaluate the safety and efficacy of G-CSF in patients with ACLF.
Methods
In this multicenter, prospective, controlled, open-label phase II study, 176 patients with ACLF (EASL-CLIF criteria) were randomized to receive G-CSF (5 μg/kg daily for the first 5 days and every third day thereafter until day 26) plus standard medical therapy (SMT) (n = 88) or SMT alone. The primary efficacy endpoint was 90-day transplant-free survival analyzed by Cox regression modeling. The key secondary endpoints were overall and transplant-free survival after 360 days, the development of ACLF-related complications, and the course of liver function scores during the entire observation period.
Results
Patients treated with G-CSF had a 90-day transplant-free survival rate of 34.1% compared to 37.5% in the SMT group (hazard ratio [HR] 1.05; 95% CI 0.711–1.551; p = 0.805). Transplant-free and overall survival at 360 days did not differ between the 2 arms (HR 0.998; 95% CI 0.697–1.430; p = 0.992 and HR 1.058; 95% CI 0.727–1.548; p = 0.768, respectively). G-CSF did not improve liver function scores, the occurrence of infections, or survival in subgroups of patients without infections, with alcohol-related ACLF, or with ACLF defined by the APASL criteria. Sixty-one serious adverse events were reported in the G-CSF+SMT group and 57 were reported in the SMT group. In total, 7 drug-related serious adverse reactions occurred in the G-CSF group. The study was prematurely terminated due to futility after conditional power calculation.
Conclusions
In contrast to previous findings, G-CSF had no significant beneficial effect on patients with ACLF in this multicenter controlled trial, which suggests that it should not be used as a standard treatment for ACLF.
ClinicalTrials.gov number
NCT02669680
Lay summary
Granulocyte-colony stimulating factor was considered as a novel treatment for acute-on-chronic liver failure (ACLF). We performed the first randomized, multicenter, controlled phase II trial, which showed that G-CSF did not improve survival or other clinical endpoints in patients with ACLF. Therefore, G-CSF should not be used to treat liver disease outside clinical studies.
中文翻译:

粒细胞集落刺激因子 (G-CSF) 治疗慢加急性肝衰竭:一项多中心随机试验(GRAFT 研究)
背景与目标
基于小型单中心研究的积极结果,粒细胞集落刺激因子 (G-CSF) 正在广泛用于治疗慢加急性肝衰竭 (ACLF) 患者。在此,我们旨在评估 G-CSF 在 ACLF 患者中的安全性和有效性。
方法
在这项多中心、前瞻性、对照、开放标签的 II 期研究中,176 名 ACLF(EASL-CLIF 标准)患者被随机分配接受 G-CSF(前 5 天每天 5 μg/kg,此后每三天一次,直到第26) 加上标准药物治疗 (SMT) (n = 88) 或单独的 SMT。主要疗效终点是通过 Cox 回归模型分析的 90 天无移植生存期。关键的次要终点是 360 天后的总体和无移植生存、ACLF 相关并发症的发展以及整个观察期间的肝功能评分过程。
结果
接受 G-CSF 治疗的患者 90 天无移植生存率为 34.1%,而 SMT 组为 37.5%(风险比 [HR] 1.05;95% CI 0.711–1.551;p = 0.805)。360 天的无移植生存期和总生存期在 2 组之间没有差异(HR 0.998;95% CI 0.697–1.430;p = 0.992 和 HR 1.058;95% CI 0.727–1.548;p =0.768,分别)。G-CSF 未改善无感染、酒精相关 ACLF 或 APASL 标准定义的 ACLF 患者亚组的肝功能评分、感染发生率或存活率。G-CSF+SMT 组报告了 61 例严重不良事件,SMT 组报告了 57 例。G-CSF组共发生7例药物相关的严重不良反应。该研究在条件功效计算后因无效而提前终止。
结论
与之前的研究结果相反,在这项多中心对照试验中,G-CSF 对 ACLF 患者没有显着的益处,这表明它不应该作为 ACLF 的标准治疗方法。
ClinicalTrials.gov 编号
NCT02669680
总结
粒细胞集落刺激因子被认为是一种治疗慢加急性肝衰竭(ACLF)的新方法。我们进行了第一项随机、多中心、对照 II 期试验,结果表明 G-CSF 并未改善 ACLF 患者的生存率或其他临床终点。因此,G-CSF 不应用于临床研究之外的肝病治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号