当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Group 14 Central Atoms and Halogen Bonding in Different Dielectric Environments: How Germanium Outperforms Silicon
ChemPlusChem ( IF 3.0 ) Pub Date : 2021-08-04 , DOI: 10.1002/cplu.202100294 Kelling J Donald 1 , Supreeth Prasad 1 , Kaitlin Wilson 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2021-08-04 , DOI: 10.1002/cplu.202100294 Kelling J Donald 1 , Supreeth Prasad 1 , Kaitlin Wilson 1
Affiliation
|
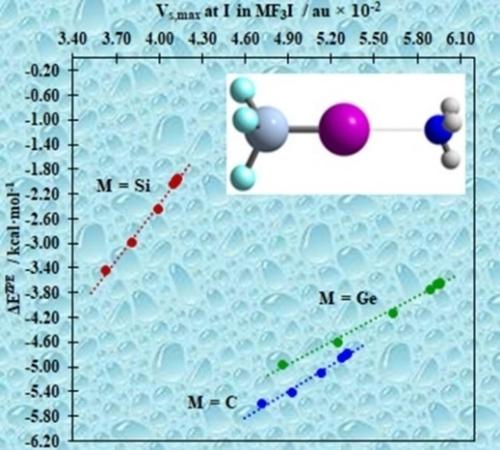
Germanium iodides can form halogen bonds energetically and structurally comparable to organic cases. Silicon analogues cannot. That, as is shown here, is not so only in the gas phase but over a wide range of permittivities. Moreover, geminal substituents on tetravalent C, Si, and Ge halides are not innocent in halogen bonding – through bond polarization and through space repulsion are crucial, accounting for apparently counterintuitive trends.

中文翻译:

不同介电环境中的 14 族中心原子和卤素键合:锗如何优于硅
碘化锗可以在能量和结构上形成与有机情况相当的卤素键。硅类似物不能。正如这里所示,这不仅在气相中而且在很宽的介电常数范围内都是如此。此外,四价 C、Si 和 Ge 卤化物上的孪晶取代基在卤素键合中并不是无害的——通过键极化和通过空间排斥是至关重要的,这显然是违反直觉的趋势。

更新日期:2021-08-04

中文翻译:

不同介电环境中的 14 族中心原子和卤素键合:锗如何优于硅
碘化锗可以在能量和结构上形成与有机情况相当的卤素键。硅类似物不能。正如这里所示,这不仅在气相中而且在很宽的介电常数范围内都是如此。此外,四价 C、Si 和 Ge 卤化物上的孪晶取代基在卤素键合中并不是无害的——通过键极化和通过空间排斥是至关重要的,这显然是违反直觉的趋势。












































 京公网安备 11010802027423号
京公网安备 11010802027423号