当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
T- and pH-Dependent Kinetics of the Reactions of ·OH(aq) with Glutaric and Adipic Acid for Atmospheric Aqueous-Phase Chemistry
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-08-05 , DOI: 10.1021/acsearthspacechem.1c00163 Liang Wen 1 , Thomas Schaefer 1 , Lin He 1 , Yimu Zhang 2 , Xiaomin Sun 3 , Oscar N. Ventura 1, 4 , Hartmut Herrmann 1, 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-08-05 , DOI: 10.1021/acsearthspacechem.1c00163 Liang Wen 1 , Thomas Schaefer 1 , Lin He 1 , Yimu Zhang 2 , Xiaomin Sun 3 , Oscar N. Ventura 1, 4 , Hartmut Herrmann 1, 2
Affiliation
|
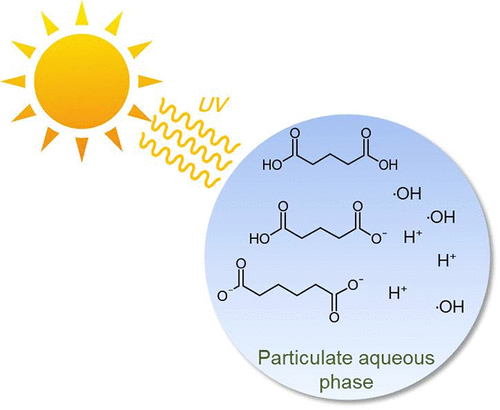
Glutaric acid and adipic acid are dicarboxylic acids (DCAs) that are commonly found in atmospheric aerosols and cloud droplets. Within this study, the temperature- and pH-dependent rate constants of aqueous-phase OH radical reactions of these two DCAs were determined through the competition kinetics method. The following Arrhenius expressions were derived for the temperature dependency of the OH radical reaction with glutaric acid: k(T, H2A) = (3.9 ± 0.1) × 1010 × exp[(−1270 ± 200 K)/T], k(T, HA–) = (2.3 ± 0.1) × 1011 × exp[(−1660 ± 190 K)/T], k(T, A2–) = (1.4 ± 0.1) × 1011 × exp[(−1400 ± 170 K)/T]. Similarly, for adipic acid: k(T, H2A) = (7.5 ± 0.2) × 1010 × exp[(−1210 ± 170 K)/T], k(T, HA–) = (9.5 ± 0.3) × 1010 × exp[(−1200 ± 200 K)/T], k(T, A2–) = (8.7 ± 0.2) × 1010 × exp[(−1100 ± 170 K)/T] (in units of L mol–1 s–1). Density functional theory (DFT) calculations were performed to calculate the energy barrier of the H atom abstraction. The calculation results show that the energy barriers at the Cβ-atoms of the two DCAs are much lower than those at the Cα-atoms, indicating that the H atom abstractions predominantly occur at the Cβ-atoms. The increased ·OH rate constant in the case of the deprotonated form can be explained by the reduction of energy barrier, which is predominately caused by the variation of the inductive effect of the carboxyl group. As an important sink in the atmosphere, the degradation of glutaric acid and adipic acid by ·OH under atmospheric conditions can be accurately described by the Arrhenius expressions obtained.
中文翻译:

常压水相化学中·OH(aq)与戊二酸和己二酸反应的T-和pH-相关动力学
戊二酸和己二酸是通常存在于大气气溶胶和云滴中的二羧酸 (DCA)。在本研究中,这两种 DCA 的水相 OH 自由基反应的温度和 pH 相关速率常数是通过竞争动力学方法确定的。对于与戊二酸的 OH 自由基反应的温度依赖性,推导出以下 Arrhenius 表达式:k ( T , H 2 A) = (3.9 ± 0.1) × 10 10 × exp[(-1270 ± 200 K)/ T ],k ( T , HA – ) = (2.3 ± 0.1) × 10 11 × exp[(−1660 ± 190 K)/ T ], k (T , A 2– ) = (1.4 ± 0.1) × 10 11 × exp[(−1400 ± 170 K)/ T ]。同样,对于己二酸:k ( T , H 2 A) = (7.5 ± 0.2) × 10 10 × exp[(−1210 ± 170 K)/ T ], k ( T , HA – ) = (9.5 ± 0.3) × 10 10 × exp[(−1200 ± 200 K)/ T ], k ( T , A 2– ) = (8.7 ± 0.2) × 10 10 × exp[(−1100 ± 170 K)/ T ](单位L mol –1 s –1)。进行密度泛函理论 (DFT) 计算以计算 H 原子抽象的能垒。计算结果表明,两个DCA的C β -原子处的能垒远低于C α -原子处的能量势垒,表明H原子提取主要发生在C β -原子处。在去质子化形式的情况下,增加的·OH速率常数可以用能垒的降低来解释,这主要是由羧基的诱导效应的变化引起的。戊二酸和己二酸作为大气中的重要汇,大气条件下·OH对戊二酸和己二酸的降解可以用得到的Arrhenius表达式准确描述。
更新日期:2021-08-19
中文翻译:

常压水相化学中·OH(aq)与戊二酸和己二酸反应的T-和pH-相关动力学
戊二酸和己二酸是通常存在于大气气溶胶和云滴中的二羧酸 (DCA)。在本研究中,这两种 DCA 的水相 OH 自由基反应的温度和 pH 相关速率常数是通过竞争动力学方法确定的。对于与戊二酸的 OH 自由基反应的温度依赖性,推导出以下 Arrhenius 表达式:k ( T , H 2 A) = (3.9 ± 0.1) × 10 10 × exp[(-1270 ± 200 K)/ T ],k ( T , HA – ) = (2.3 ± 0.1) × 10 11 × exp[(−1660 ± 190 K)/ T ], k (T , A 2– ) = (1.4 ± 0.1) × 10 11 × exp[(−1400 ± 170 K)/ T ]。同样,对于己二酸:k ( T , H 2 A) = (7.5 ± 0.2) × 10 10 × exp[(−1210 ± 170 K)/ T ], k ( T , HA – ) = (9.5 ± 0.3) × 10 10 × exp[(−1200 ± 200 K)/ T ], k ( T , A 2– ) = (8.7 ± 0.2) × 10 10 × exp[(−1100 ± 170 K)/ T ](单位L mol –1 s –1)。进行密度泛函理论 (DFT) 计算以计算 H 原子抽象的能垒。计算结果表明,两个DCA的C β -原子处的能垒远低于C α -原子处的能量势垒,表明H原子提取主要发生在C β -原子处。在去质子化形式的情况下,增加的·OH速率常数可以用能垒的降低来解释,这主要是由羧基的诱导效应的变化引起的。戊二酸和己二酸作为大气中的重要汇,大气条件下·OH对戊二酸和己二酸的降解可以用得到的Arrhenius表达式准确描述。











































 京公网安备 11010802027423号
京公网安备 11010802027423号