Progress in Nuclear Energy ( IF 3.3 ) Pub Date : 2021-08-05 , DOI: 10.1016/j.pnucene.2021.103887 Pegah Khajavi 1 , Ali Reza Keshtkar 2 , Mohammad Ali Moosavian 1
|
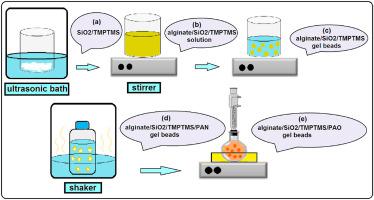
Adsorption of U(VI) ions from aqueous solution onto a novel amidoximated modified calcium alginate bead with entrapped functionalized SiO2 nanoparticles, (alginate/SiO2/TMPTMS/PAO), with variation in SiO2 and 3-mercaptopropyltrimethoxysilane (TMPTMS) contents was studied. In order to synthesis of the composite adsorbent, SiO2 nanoparticles were first modified via TMPTMS and then applied to prepare the alginate gel beads. The adsorption capacity of U(VI) ions by alginate/SiO2/TMPTMS/PAO beads at 5 wt % of SiO2 and 30 wt % of TMPTMS (with respect to the alginate weight) was 3.6 times greater than that of the blank one (alginate/PAO). The characterizations of adsorbent were carried out using FTIR, SEM-EDX and BET analyses. The equilibrium and kinetic data were well described by the Langmuir isotherm and Pseudo-second order kinetic models, respectively. The effect of operating variables, including initial pH of solution, initial concentration of U(VI) solution and the dosage of adsorbent on the adsorption capacity of uranium ions were investigated by central composite design (CCD) of response surface methodology (RSM). The effect of presence of copper, cadmium and nickel ions on the adsorption of uranium ions in binary systems was investigated. In this work, for the first time, modified SiO2 nanoparticles with –SH group, have been added to the calcium-alginate gel beads to increase the mechanical, thermal and radiative resistance of alginate as well as to increase the uranium adsorption capacity. The results showed that alginate/SiO2/TMPTMS/PAO composite adsorbent was effective in removal of U(VI) ions from aqueous solutions.
中文翻译:

一种新型酰胺肟化改性海藻酸钙凝胶珠对 U(VI) 去除的优化,其中包含包埋的功能化 SiO2 纳米颗粒
U(VI) 离子从水溶液吸附到新的酰胺肟化改性海藻酸钙珠上,其中包埋了功能化的 SiO 2纳米颗粒(海藻酸盐/SiO 2 /TMPTMS/PAO),SiO 2和 3-巯基丙基三甲氧基硅烷 (TMPTMS) 的含量发生变化学习了。为了合成复合吸附剂,首先通过TMPTMS对SiO 2纳米粒子进行改性,然后将其用于制备海藻酸盐凝胶珠。海藻酸盐/SiO 2 /TMPTMS/PAO 珠在 5 wt % SiO 2 下对 U(VI) 离子的吸附能力30 wt% 的 TMPTMS(相对于藻酸盐重量)是空白样品(藻酸盐/PAO)的 3.6 倍。使用 FTIR、SEM-EDX 和 BET 分析对吸附剂进行表征。Langmuir 等温线和伪二级动力学模型分别很好地描述了平衡和动力学数据。采用响应面法(RSM)的中心复合设计(CCD)研究了溶液初始pH值、U(VI)溶液初始浓度和吸附剂用量等操作变量对铀离子吸附容量的影响。研究了铜、镉和镍离子的存在对二元系统中铀离子吸附的影响。在这项工作中,首次将改性 SiO 2具有–SH 基团的纳米粒子已被添加到海藻酸钙凝胶珠中,以增加海藻酸盐的机械、热和辐射阻力,并增加铀的吸附能力。结果表明,海藻酸盐/SiO 2 /TMPTMS/PAO复合吸附剂可有效去除水溶液中的U(VI)离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号