Translational Oncology ( IF 4.5 ) Pub Date : 2021-08-05 , DOI: 10.1016/j.tranon.2021.101191 Sai-Hong Ignatius Ou 1 , Misako Nagasaka 2 , Danielle Brazel 3 , Yujie Hou 4 , Viola W Zhu 1
|
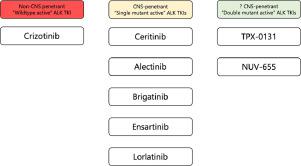
Our current treatment paradigm of advanced anaplastic lymphoma kinase fusion (ALK+) non-small cell lung cancer (NSCLC) classifies the six currently approved ALK tyrosine kinase inhibitors (TKIs) into three generations. The 2nd-generation (2G) and 3rd-generation (3G) ALK TKIs are all “single mutant active” with varying potencies across a wide spectrum of acquired single ALK resistance mutations. There is a vigorous debate among clinicians which is the best upfront ALK TKI is for the first-line (1L) treatment of ALK+ NSCLC and the subsequent sequencing strategies whether it should be based on the presence of specific on-target ALK resistance mutations or not. Regardless, sequential use of “single mutant active” ALK TKIs will eventually lead to double ALK resistance mutations in cis. This has led to the creation of fourth generation (4G) “double mutant active” ALK TKIs such as TPX-0131 and NVL-655. We discuss the critical properties 4G ALK TKIs must possess to be clinically successful. We proposed conceptual first-line, second-line, and molecularly-based third-line registrational randomized clinical trials designed for these 4G ALK TKIs. How these 4G ALK TKIs would be used in the future will depend on which line of treatment the clinical trial design(s) is adopted provided the trial is positive. If approved, 4G ALK TKIs may usher in a new treatment paradigm for advanced ALK+ NSCLC that is based on classifying ALK TKIs based on the intrinsic functional capabilities (“singe mutant active” versus “double mutant active”) rather than the loosely-defined “generational” (first-, second-,third-,fourth-) classification and avoid the current clinical approaches of seemingly random sequential use of 2G and 3G ALK TKIs.
中文翻译:

第四代“双突变活性”ALK TKI(TPX-0131和NVL-655)的临床开发是否会改变ALK+ NSCLC的未来治疗范式?
我们目前的晚期间变性淋巴瘤激酶融合 ( ALK+ ) 非小细胞肺癌 (NSCLC) 治疗范例将目前批准的六种 ALK 酪氨酸激酶抑制剂 (TKI) 分为三代。第二代 (2G) 和第三代 (3G) ALK TKI 均具有“单突变活性”,在广泛的获得性单ALK耐药突变中具有不同的效力。临床医生之间存在着激烈的争论:对于ALK + NSCLC 的一线 (1L) 治疗,最好的前期 ALK TKI 是什么?后续的测序策略是否应该基于特定的靶向ALK耐药突变的存在或不是。无论如何,连续使用“单突变活性”ALK TKI 最终将导致顺式双ALK耐药突变。这导致了第四代 (4G)“双突变活性”ALK TKI 的创建,例如 TPX-0131 和 NVL-655。我们讨论 4G ALK TKI 要想在临床上取得成功必须具备的关键特性。我们提出了针对这些 4G ALK TKI 设计的概念性一线、二线和基于分子的三线注册随机临床试验。这些 4G ALK TKI 未来如何使用将取决于临床试验设计采用哪一种治疗方案(如果试验结果呈阳性)。 如果获得批准,4G ALK TKI 可能会为晚期ALK+ NSCLC 带来新的治疗范式,该范式基于内在功能能力(“单突变活性”与“双突变活性”)而不是松散定义的“代”(第一、第二、第三、第四)分类,并避免目前看似随机顺序使用 2G 和 3G ALK TKI 的临床方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号