Applied Surface Science ( IF 6.3 ) Pub Date : 2021-08-04 , DOI: 10.1016/j.apsusc.2021.150831 Wenxin Zhu 1 , Xiong Chang 1 , Ding Ding 1 , Changsong Zhou 1 , Hao Wu 1 , Zhen Zhang 1 , Hongmin Yang 1 , Lushi Sun 2 , Yaming Zhou 3
|
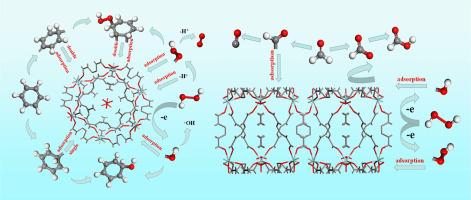
Formaldehyde and benzene adsorption on UIO-66 in coordination with gaseous H2O2 is an efficient and recyclable method for removing formaldehyde and benzene. In this study, the interaction mechanism of formaldehyde and benzene coordinated with UIO-66 under the existence of H2O2 was studied using density functional theory. The results showed that the decomposition of H2O2 by UIO-66 was promoted to produce highly oxidizing hydroxyl groups. The structure of formaldehyde obviously changed in H2O2/UIO-66 system after equilibrium. The C H bond of HCHO molecule was easy to break at different adsorption sites of UIO-66. Comparatively, benzene with distorted ring structure tended to chemically bound to the carbon site of UIO-66, but the decomposition of benzene was difficult. Density of states analysis showed that the adsorption between C6H6 and UIO-66 was chemisorption. The study is of great significance for providing theoretical guidance to the removal of organic pollutants from exhaust gas by UIO-66.
H bond of HCHO molecule was easy to break at different adsorption sites of UIO-66. Comparatively, benzene with distorted ring structure tended to chemically bound to the carbon site of UIO-66, but the decomposition of benzene was difficult. Density of states analysis showed that the adsorption between C6H6 and UIO-66 was chemisorption. The study is of great significance for providing theoretical guidance to the removal of organic pollutants from exhaust gas by UIO-66.
中文翻译:

使用密度泛函理论与气态H2O2配位在UIO-66上吸附甲醛和苯的机理
与气态H 2 O 2配合在UIO-66 上吸附甲醛和苯是去除甲醛和苯的有效且可回收的方法。本研究利用密度泛函理论研究了在H 2 O 2存在下甲醛和苯与UIO-66配位的相互作用机理。结果表明,UIO-66促进了H 2 O 2的分解,产生了高氧化性的羟基。平衡后甲醛在H 2 O 2 /UIO-66体系中的结构发生明显变化。C HCHO分子的H键在UIO-66的不同吸附位点容易断裂。相比之下,环结构扭曲的苯倾向于化学键合到 UIO-66 的碳位,但苯的分解很困难。态密度分析表明C 6 H 6与UIO-66之间的吸附是化学吸附。该研究对UIO-66去除废气中有机污染物提供理论指导具有重要意义。
HCHO分子的H键在UIO-66的不同吸附位点容易断裂。相比之下,环结构扭曲的苯倾向于化学键合到 UIO-66 的碳位,但苯的分解很困难。态密度分析表明C 6 H 6与UIO-66之间的吸附是化学吸附。该研究对UIO-66去除废气中有机污染物提供理论指导具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号