当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molybdenum(VI) Sequestration Mechanisms During Iron(II)-Induced Ferrihydrite Transformation
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-08-04 , DOI: 10.1021/acsearthspacechem.1c00152 Valerie A. Schoepfer 1 , Jullieta E. Lum 1 , Matthew B. J. Lindsay 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-08-04 , DOI: 10.1021/acsearthspacechem.1c00152 Valerie A. Schoepfer 1 , Jullieta E. Lum 1 , Matthew B. J. Lindsay 1
Affiliation
|
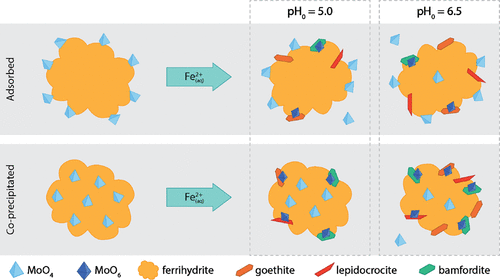
Adsorption and coprecipitation reactions with Fe(III) (oxyhydr)oxides contribute to Mo(VI) attenuation within geohydrologic systems. Redox transitions within these systems can promote transformation of metastable phases, including ferrihydrite, and repartitioning of associated Mo(VI). Recent studies show that Mo(VI) coordination shifts from tetrahedral to octahedral during Fe(II)-induced ferrihydrite transformation. However, effects of initial conditions including solution pH, the Mo(VI) uptake mechanism, and Mo(VI) loading on repartitioning are not known. We performed batch experiments using ferrihydrite suspensions prepared with adsorbed or coprecipitated Mo(VI) (0, 25, and 100 μmol g–1) at two initial pH values (pH0; 5.0 and 6.5). We catalyzed ferrihydrite transformation under anoxic conditions by adding Fe(II)(aq) (0.5 mM) and monitored pH, [Mo]T, and [Fe]T over time. After 168 h, we collected reacted solids for analysis by powder X-ray diffraction (XRD), transmission electron microscopy-selected area electron diffraction (TEM-SAED), and Mo K-edge extended X-ray absorption fine structure (EXAFS) spectroscopy. XRD data indicate that bulk ferrihydrite transformation was limited in all but the pH0 6.5 coprecipitated Mo(VI) experiments. The TEM-SAED results reveal that nanoscale lepidocrocite and goethite formed at ferrihydrite surfaces in all experiments, whereas nanoscale bamfordite [FeMo2O6(OH)3·H2O] crystallites were observed in pH0 6.5 experiments. EXAFS models reveal changes in Mo(VI) coordination and bonding consistent with bamfordite precipitation combined with structural incorporation into neoformed goethite and lepidocrocite. Our results improve the understanding of Mo(VI) retention pathways in geohydrologic systems.
中文翻译:

铁 (II) 诱导的水铁矿转化过程中的钼 (VI) 螯合机制
与 Fe(III)(羟基)氧化物的吸附和共沉淀反应会导致地质水文系统内的 Mo(VI) 衰减。这些系统内的氧化还原转变可以促进亚稳态相的转变,包括水铁矿,以及相关 Mo(VI) 的重新分配。最近的研究表明,在 Fe(II) 诱导的水铁矿转变过程中,Mo(VI) 的配位从四面体转变为八面体。然而,包括溶液 pH 值、Mo(VI) 吸收机制和 Mo(VI) 负载对重新分配的初始条件的影响尚不清楚。我们使用由吸附或共沉淀的 Mo(VI)(0、25 和 100 μmol g –1)在两个初始 pH 值(pH 0; 5.0 和 6.5)。我们通过添加 Fe(II) (aq) (0.5 mM)在缺氧条件下催化水铁矿转化,并随时间监测 pH、[Mo] T和 [Fe] T。168 小时后,我们收集了反应后的固体,用于粉末 X 射线衍射 (XRD)、透射电子显微镜选区电子衍射 (TEM-SAED) 和 Mo K 边缘扩展 X 射线吸收精细结构 (EXAFS) 光谱分析. XRD 数据表明,除了 pH 0 6.5 的共沉淀 Mo(VI) 实验外,整体水铁矿转变受到限制。TEM-SAED 结果表明,在所有实验中,在水铁矿表面形成了纳米级纤铁矿和针铁矿,而纳米级菱镁矿 [FeMo 2 O 6(OH) 3 ·H 2 O] 微晶在 pH 0 6.5 实验中观察到。EXAFS 模型揭示了 Mo(VI) 配位和键合的变化,这与菱镁矿沉淀相结合,并结合了新形成的针铁矿和纤铁矿的结构。我们的结果提高了对地质水文系统中 Mo(VI) 保留途径的理解。
更新日期:2021-08-19
中文翻译:

铁 (II) 诱导的水铁矿转化过程中的钼 (VI) 螯合机制
与 Fe(III)(羟基)氧化物的吸附和共沉淀反应会导致地质水文系统内的 Mo(VI) 衰减。这些系统内的氧化还原转变可以促进亚稳态相的转变,包括水铁矿,以及相关 Mo(VI) 的重新分配。最近的研究表明,在 Fe(II) 诱导的水铁矿转变过程中,Mo(VI) 的配位从四面体转变为八面体。然而,包括溶液 pH 值、Mo(VI) 吸收机制和 Mo(VI) 负载对重新分配的初始条件的影响尚不清楚。我们使用由吸附或共沉淀的 Mo(VI)(0、25 和 100 μmol g –1)在两个初始 pH 值(pH 0; 5.0 和 6.5)。我们通过添加 Fe(II) (aq) (0.5 mM)在缺氧条件下催化水铁矿转化,并随时间监测 pH、[Mo] T和 [Fe] T。168 小时后,我们收集了反应后的固体,用于粉末 X 射线衍射 (XRD)、透射电子显微镜选区电子衍射 (TEM-SAED) 和 Mo K 边缘扩展 X 射线吸收精细结构 (EXAFS) 光谱分析. XRD 数据表明,除了 pH 0 6.5 的共沉淀 Mo(VI) 实验外,整体水铁矿转变受到限制。TEM-SAED 结果表明,在所有实验中,在水铁矿表面形成了纳米级纤铁矿和针铁矿,而纳米级菱镁矿 [FeMo 2 O 6(OH) 3 ·H 2 O] 微晶在 pH 0 6.5 实验中观察到。EXAFS 模型揭示了 Mo(VI) 配位和键合的变化,这与菱镁矿沉淀相结合,并结合了新形成的针铁矿和纤铁矿的结构。我们的结果提高了对地质水文系统中 Mo(VI) 保留途径的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号