Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simultaneous biochemical and functional phenotyping of single circulating tumor cells using ultrahigh throughput and recovery microfluidic devices
Lab on a Chip ( IF 6.1 ) Pub Date : 2021-07-26 , DOI: 10.1039/d1lc00454a Yang Liu 1 , Wujun Zhao 2 , Rui Cheng 3 , Jamie Hodgson 4 , Mary Egan 4 , Christen N Cooper Pope 4 , Petros G Nikolinakos 4 , Leidong Mao 3
Lab on a Chip ( IF 6.1 ) Pub Date : 2021-07-26 , DOI: 10.1039/d1lc00454a Yang Liu 1 , Wujun Zhao 2 , Rui Cheng 3 , Jamie Hodgson 4 , Mary Egan 4 , Christen N Cooper Pope 4 , Petros G Nikolinakos 4 , Leidong Mao 3
Affiliation
|
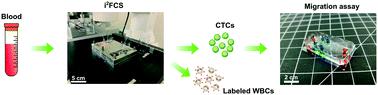
Profiling circulating tumour cells (CTCs) in cancer patients' blood samples is critical to understand the complex and dynamic nature of metastasis. This task is challenged by the fact that CTCs are not only extremely rare in circulation but also highly heterogeneous in their molecular programs and cellular functions. Here we report a combinational approach for the simultaneous biochemical and functional phenotyping of patient-derived CTCs, using an integrated inertial ferrohydrodynamic cell separation (i2FCS) method and a single-cell microfluidic migration assay. This combinatorial approach offers unique capability to profile CTCs on the basis of their surface expression and migratory characteristics. We achieve this using the i2FCS method that successfully processes whole blood samples in a tumor cell marker and size agnostic manner. The i2FCS method enables an ultrahigh blood sample processing throughput of up to 2 × 105 cells s−1 with a blood sample flow rate of 60 mL h−1. Its short processing time (10 minutes for a 10 mL sample), together with a close-to-complete CTC recovery (99.70% recovery rate) and a low WBC contamination (4.07-log depletion rate by removing 99.992% of leukocytes), results in adequate and functional CTCs for subsequent studies in the single-cell migration device. For the first time, we employ this new approach to query CTCs with single-cell resolution in accordance with their expression of phenotypic surface markers and migration properties, revealing the dynamic phenotypes and the existence of a high-motility subpopulation of CTCs in blood samples from metastatic lung cancer patients. This method could be adopted to study the biological and clinical value of invasive CTC phenotypes.
中文翻译:

使用超高通量和恢复微流体装置同时对单个循环肿瘤细胞进行生化和功能表型分析
分析癌症患者血液样本中的循环肿瘤细胞 (CTC) 对于了解转移的复杂性和动态性至关重要。这项任务受到以下事实的挑战:CTC 不仅在循环中极为罕见,而且在其分子程序和细胞功能方面也高度异质。在这里,我们报告了一种使用集成惯性铁水动力细胞分离 (i 2 FCS) 方法和单细胞微流体迁移测定对患者衍生的 CTC 进行同步生化和功能表型的组合方法。这种组合方法提供了独特的能力,可以根据 CTC 的表面表达和迁移特性对其进行分析。我们使用 i 2来实现这一点FCS 方法以肿瘤细胞标记物和大小不可知的方式成功处理全血样本。i 2 FCS 方法可实现高达 2 × 10 5 个细胞 s -1的超高血样处理通量,血样流速为 60 mL h -1. 其处理时间短(10 mL 样品为 10 分钟),加上接近完全的 CTC 回收率(99.70% 回收率)和低 WBC 污染(去除 99.992% 的白细胞,4.07-log 消耗率),结果在足够的和功能的 CTC 中用于单细胞迁移装置的后续研究。我们首次采用这种新方法,根据表型表面标志物的表达和迁移特性,以单细胞分辨率查询 CTC,揭示动态表型和血液样本中 CTC 高运动亚群的存在。转移性肺癌患者。该方法可用于研究侵袭性 CTC 表型的生物学和临床价值。
更新日期:2021-08-04
中文翻译:

使用超高通量和恢复微流体装置同时对单个循环肿瘤细胞进行生化和功能表型分析
分析癌症患者血液样本中的循环肿瘤细胞 (CTC) 对于了解转移的复杂性和动态性至关重要。这项任务受到以下事实的挑战:CTC 不仅在循环中极为罕见,而且在其分子程序和细胞功能方面也高度异质。在这里,我们报告了一种使用集成惯性铁水动力细胞分离 (i 2 FCS) 方法和单细胞微流体迁移测定对患者衍生的 CTC 进行同步生化和功能表型的组合方法。这种组合方法提供了独特的能力,可以根据 CTC 的表面表达和迁移特性对其进行分析。我们使用 i 2来实现这一点FCS 方法以肿瘤细胞标记物和大小不可知的方式成功处理全血样本。i 2 FCS 方法可实现高达 2 × 10 5 个细胞 s -1的超高血样处理通量,血样流速为 60 mL h -1. 其处理时间短(10 mL 样品为 10 分钟),加上接近完全的 CTC 回收率(99.70% 回收率)和低 WBC 污染(去除 99.992% 的白细胞,4.07-log 消耗率),结果在足够的和功能的 CTC 中用于单细胞迁移装置的后续研究。我们首次采用这种新方法,根据表型表面标志物的表达和迁移特性,以单细胞分辨率查询 CTC,揭示动态表型和血液样本中 CTC 高运动亚群的存在。转移性肺癌患者。该方法可用于研究侵袭性 CTC 表型的生物学和临床价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号