当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
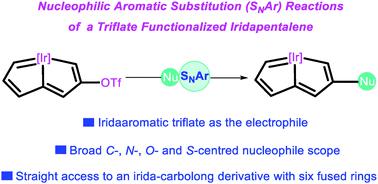
Carbolong chemistry: nucleophilic aromatic substitution of a triflate functionalized iridapentalene
Chemical Communications ( IF 4.9 ) Pub Date : 2021-07-30 , DOI: 10.1039/d1cc03261e Jinhua Li 1 , Zhengyu Lu , Yuhui Hua , Dafa Chen , Haiping Xia
Chemical Communications ( IF 4.9 ) Pub Date : 2021-07-30 , DOI: 10.1039/d1cc03261e Jinhua Li 1 , Zhengyu Lu , Yuhui Hua , Dafa Chen , Haiping Xia
Affiliation
|
The reactivity of the triflate functionalized iridapentalene 1, [Ir{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH2C(CO2Me)2CH2)
CHC(CH2C(CO2Me)2CH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC
CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(OTf)
CHC(OTf)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH}(CO)(PPh3)2]OTf, with C-, N-, O- and S-centered neutral nucleophiles was studied, leading to the isolation of a wide array of irida–carbolong derivatives. As an extension, a polycyclic complex with a rare six-fused-ring structure was constructed. This strategy provides a new route for the construction of functionalized metallaaromatic complexes, and the resulting iridacycles exhibit broad spectral absorption ranges, making them potential photoelectric materials.
CH}(CO)(PPh3)2]OTf, with C-, N-, O- and S-centered neutral nucleophiles was studied, leading to the isolation of a wide array of irida–carbolong derivatives. As an extension, a polycyclic complex with a rare six-fused-ring structure was constructed. This strategy provides a new route for the construction of functionalized metallaaromatic complexes, and the resulting iridacycles exhibit broad spectral absorption ranges, making them potential photoelectric materials.
中文翻译:

Carbolong 化学:三氟甲磺酸酯官能化的 iridapentalene 的亲核芳香取代
三氟甲磺酸酯官能化的 iridapentalene 1, [Ir{![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH 2 C(CO 2 Me) 2 CH 2 )
CHC(CH 2 C(CO 2 Me) 2 CH 2 ) ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC
CC ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(OTf)
CHC(OTf) ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH}(CO)(PPh 3 ) 2 ]OTf 与C -、N - 的反应性, O - 和S对中心中性亲核试剂进行了研究,从而分离了多种 irida-carbolong 衍生物。作为扩展,构建了具有稀有六稠环结构的多环复合物。该策略为功能化金属芳香族配合物的构建提供了一条新途径,由此产生的虹彩环具有广泛的光谱吸收范围,使其成为潜在的光电材料。
CH}(CO)(PPh 3 ) 2 ]OTf 与C -、N - 的反应性, O - 和S对中心中性亲核试剂进行了研究,从而分离了多种 irida-carbolong 衍生物。作为扩展,构建了具有稀有六稠环结构的多环复合物。该策略为功能化金属芳香族配合物的构建提供了一条新途径,由此产生的虹彩环具有广泛的光谱吸收范围,使其成为潜在的光电材料。
更新日期:2021-08-04
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH2C(CO2Me)2CH2)
CHC(CH2C(CO2Me)2CH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC
CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(OTf)
CHC(OTf)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH}(CO)(PPh3)2]OTf, with C-, N-, O- and S-centered neutral nucleophiles was studied, leading to the isolation of a wide array of irida–carbolong derivatives. As an extension, a polycyclic complex with a rare six-fused-ring structure was constructed. This strategy provides a new route for the construction of functionalized metallaaromatic complexes, and the resulting iridacycles exhibit broad spectral absorption ranges, making them potential photoelectric materials.
CH}(CO)(PPh3)2]OTf, with C-, N-, O- and S-centered neutral nucleophiles was studied, leading to the isolation of a wide array of irida–carbolong derivatives. As an extension, a polycyclic complex with a rare six-fused-ring structure was constructed. This strategy provides a new route for the construction of functionalized metallaaromatic complexes, and the resulting iridacycles exhibit broad spectral absorption ranges, making them potential photoelectric materials.
中文翻译:

Carbolong 化学:三氟甲磺酸酯官能化的 iridapentalene 的亲核芳香取代
三氟甲磺酸酯官能化的 iridapentalene 1, [Ir{
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH 2 C(CO 2 Me) 2 CH 2 )
CHC(CH 2 C(CO 2 Me) 2 CH 2 ) ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC
CC ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(OTf)
CHC(OTf) ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH}(CO)(PPh 3 ) 2 ]OTf 与C -、N - 的反应性, O - 和S对中心中性亲核试剂进行了研究,从而分离了多种 irida-carbolong 衍生物。作为扩展,构建了具有稀有六稠环结构的多环复合物。该策略为功能化金属芳香族配合物的构建提供了一条新途径,由此产生的虹彩环具有广泛的光谱吸收范围,使其成为潜在的光电材料。
CH}(CO)(PPh 3 ) 2 ]OTf 与C -、N - 的反应性, O - 和S对中心中性亲核试剂进行了研究,从而分离了多种 irida-carbolong 衍生物。作为扩展,构建了具有稀有六稠环结构的多环复合物。该策略为功能化金属芳香族配合物的构建提供了一条新途径,由此产生的虹彩环具有广泛的光谱吸收范围,使其成为潜在的光电材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号