当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photolysis of 3-Azido-3-phenyl-3H-isobenzofuran-1-one at Ambient and Cryogenic Temperatures†
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2021-08-04 , DOI: 10.1111/php.13500 Kosala Thenna-Hewa 1 , William Sebastien 1 , Elaine M Lemen 1 , William L Karney 2 , Manabu Abe 3 , Anna D Gudmundsdottir 1
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2021-08-04 , DOI: 10.1111/php.13500 Kosala Thenna-Hewa 1 , William Sebastien 1 , Elaine M Lemen 1 , William L Karney 2 , Manabu Abe 3 , Anna D Gudmundsdottir 1
Affiliation
|
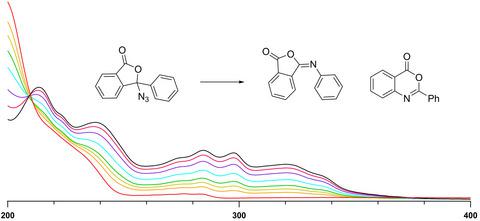
Although alkyl azides are known to typically form imines under direct irradiation, the product formation mechanism remains ambiguous as some alkyl azides also yield the corresponding triplet alkylnitrenes at cryogenic temperatures. The photoreactivity of 3-azido-3-phenyl-3H-isobenzofuran-1-one (1) was investigated in solution and in cryogenic matrices. Irradiation (λ = 254 nm) of azide 1 in acetonitrile yielded a mixture of imines 2 and 3. Monitoring of the reaction progress using UV-Vis absorption spectroscopy revealed an isosbestic point at 210 nm, indicating that the reaction proceeded cleanly. Similar results were observed for the photoreactivity of azide 1 in a frozen 2-methyltetrahydrofuran (mTHF) matrix. Irradiation of azide 1 in an argon matrix at 6 K resulted in the disappearance of its IR bands with the concurrent appearance of IR bands corresponding to imines 2 and 3. Thus, it was theorized that azide 1 forms imines 2 and 3 via a concerted mechanism from its singlet excited state or through singlet alkylnitrene 11N, which does not intersystem cross to its triplet configuration. This proposal was supported by CASPT2 calculations on a model system, which suggested that the energy gap between the singlet and triplet configurations of alkylnitrene 1N is 33 kcal/mol, thus making intersystem crossing inefficient.
中文翻译:

3-Azido-3-phenyl-3H-isobenzofuran-1-one 在环境和低温下的光解†
尽管已知烷基叠氮化物通常在直接照射下形成亚胺,但产物形成机制仍然不明确,因为一些烷基叠氮化物也在低温下产生相应的三重态烷基氮烯。3-叠氮基-3-苯基-3 H的光反应性-isobenzofuran-1-one (1) 在溶液和低温基质中进行了研究。叠氮化物 1 在乙腈中的辐照 (λ = 254 nm) 产生亚胺 2 和 3 的混合物。使用 UV-Vis 吸收光谱监测反应进程揭示了 210 nm 处的等吸光点,表明反应进行得很干净。对于叠氮化物 1 在冷冻 2-甲基四氢呋喃 (mTHF) 基质中的光反应性,观察到了类似的结果。在 6 K 下辐照氩基中的叠氮化物 1 导致其 IR 波段消失,同时出现对应于亚胺 2 和 3 的 IR 波段。因此,理论上叠氮化物 1 通过协同机制形成亚胺 2 和 3从其单重激发态或通过单重烷基氮烯11N,它不会跨系统交叉到其三重配置。该提议得到了模型系统上的 CASPT2 计算的支持,该计算表明烷基氮烯 1N 的单线态和三线态构型之间的能隙为 33 kcal/mol,从而使系统间交叉效率低下。
更新日期:2021-08-04
中文翻译:

3-Azido-3-phenyl-3H-isobenzofuran-1-one 在环境和低温下的光解†
尽管已知烷基叠氮化物通常在直接照射下形成亚胺,但产物形成机制仍然不明确,因为一些烷基叠氮化物也在低温下产生相应的三重态烷基氮烯。3-叠氮基-3-苯基-3 H的光反应性-isobenzofuran-1-one (1) 在溶液和低温基质中进行了研究。叠氮化物 1 在乙腈中的辐照 (λ = 254 nm) 产生亚胺 2 和 3 的混合物。使用 UV-Vis 吸收光谱监测反应进程揭示了 210 nm 处的等吸光点,表明反应进行得很干净。对于叠氮化物 1 在冷冻 2-甲基四氢呋喃 (mTHF) 基质中的光反应性,观察到了类似的结果。在 6 K 下辐照氩基中的叠氮化物 1 导致其 IR 波段消失,同时出现对应于亚胺 2 和 3 的 IR 波段。因此,理论上叠氮化物 1 通过协同机制形成亚胺 2 和 3从其单重激发态或通过单重烷基氮烯11N,它不会跨系统交叉到其三重配置。该提议得到了模型系统上的 CASPT2 计算的支持,该计算表明烷基氮烯 1N 的单线态和三线态构型之间的能隙为 33 kcal/mol,从而使系统间交叉效率低下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号