当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of 2-R-Naphtho[2,3-b]thiophene-4,9-diones via Cyclization of 2-(R-Ethynyl)-1,4-naphthoquinones with Na2S2O3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.joc.1c00852 Denis S Baranov 1 , Alexander A Popov 1 , Danil A Nevostruev 1 , Alexey A Dmitriev 1 , Yurii V Gatilov 2 , Elena S Kobeleva 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.joc.1c00852 Denis S Baranov 1 , Alexander A Popov 1 , Danil A Nevostruev 1 , Alexey A Dmitriev 1 , Yurii V Gatilov 2 , Elena S Kobeleva 1
Affiliation
|
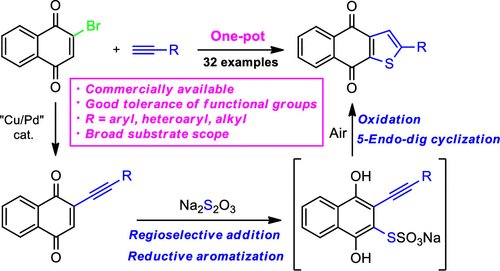
The concise and efficient one-pot synthesis of 2-R-naphtho[2,3-b]thiophene-4,9-diones from 2-bromo-1,4-naphthoquinone and alkynes has been developed. The reaction proceeds through the formation of 2-(R-ethynyl)-1,4-naphthoquinones, which undergo transformation with Na2S2O3 to 2-R-naphtho[2,3-b]thiophene-4,9-diones via C–H sulfuration, accompanied by the formation of the aromatic Bunte salt, followed by its air oxidation and 5-endo-dig cyclization. The protocol is characterized by simplicity, good tolerance for functional groups, relatively mild conditions, and commercially available starting compounds.
中文翻译:

通过 Na2S2O3 环化 2-(R-Ethynyl)-1,4-naphthoquinones 一锅法合成 2-R-Naphtho[2,3-b]thiophene-4,9-diones
已经开发了从 2-溴-1,4-萘醌和炔烃中简明高效地一锅法合成 2- R-萘并[2,3 - b ]噻吩-4,9-二酮的方法。该反应通过形成 2-( R -乙炔基)-1,4-萘醌,其与 Na 2 S 2 O 3转化为 2- R -萘并[2,3- b ]噻吩-4,9-二酮通过 C-H 硫化,伴随着芳香族 Bunte 盐的形成,然后是空气氧化和 5-endo-dig 环化。该协议的特点是简单、对官能团具有良好的耐受性、相对温和的条件和市售的起始化合物。
更新日期:2021-09-03
中文翻译:

通过 Na2S2O3 环化 2-(R-Ethynyl)-1,4-naphthoquinones 一锅法合成 2-R-Naphtho[2,3-b]thiophene-4,9-diones
已经开发了从 2-溴-1,4-萘醌和炔烃中简明高效地一锅法合成 2- R-萘并[2,3 - b ]噻吩-4,9-二酮的方法。该反应通过形成 2-( R -乙炔基)-1,4-萘醌,其与 Na 2 S 2 O 3转化为 2- R -萘并[2,3- b ]噻吩-4,9-二酮通过 C-H 硫化,伴随着芳香族 Bunte 盐的形成,然后是空气氧化和 5-endo-dig 环化。该协议的特点是简单、对官能团具有良好的耐受性、相对温和的条件和市售的起始化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号