当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pd-Catalyzed Alkene Diamination Reactions with O-Benzoylhydroxylamine Electrophiles: Evidence Supporting a Pd(II/IV) Catalytic Cycle, the Role of 2,4-Pentanedione Derivatives as Ligands, and Expanded Substrate Scope
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.joc.1c00877 Janelle K Kirsch 1 , Gabriel A Gonzalez 1 , Mason S Faculak 1 , John P Wolfe 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.joc.1c00877 Janelle K Kirsch 1 , Gabriel A Gonzalez 1 , Mason S Faculak 1 , John P Wolfe 1
Affiliation
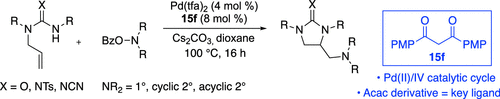
|
This article describes continued studies on Pd-catalyzed alkene diamination reactions between N-allylguanidines or ureas and O-benzoylhydroxylamine derivatives, which serve as N-centered electrophiles. The transformations generate cyclic guanidines and ureas bearing dialkylaminomethyl groups in moderate to good yield. We describe new mechanistic experiments that have led to a revised mechanistic hypothesis that involves a key oxidative addition of the electrophile to a PdII complex, followed by reductive elimination from PdIV to form the alkyl carbon–nitrogen bond. In addition, we demonstrate that acac, not phosphine, serves as a key ligand for palladium. Moreover, simple acac derivatives bearing substituted aryl groups outperform acac in the catalytic reactions, and phosphines inhibit catalysis in many cases. These discoveries have led to a significant expansion in the scope of this chemistry, which now allows for the coupling of a variety of cyclic amines, acyclic secondary amines, and primary amines. In addition, we also demonstrate that these new conditions allow for the use of amide nucleophiles, in addition to guanidines and ureas.
中文翻译:

Pd 催化烯烃二胺化反应与 O-苯甲酰羟胺亲电子试剂:支持 Pd(II/IV) 催化循环的证据、2,4-戊二酮衍生物作为配体的作用以及扩大的底物范围
本文描述了对作为 N 中心亲电试剂的N-烯丙基胍或脲与O-苯甲酰羟胺衍生物之间 Pd 催化的烯烃二化反应的持续研究。这些转化以中等至良好的产率产生了带有二烷基氨基甲基的环状胍和脲。我们描述了新的机制实验,这些实验导致了修正的机制假设,该假设涉及将亲电试剂关键的氧化添加到 Pd II复合物中,然后从 Pd IV中还原消除形成烷基碳-氮键。此外,我们证明了 acac,而不是磷化氢,是钯的关键配体。此外,带有取代芳基的简单 acac 衍生物在催化反应中的性能优于 acac,而膦在许多情况下会抑制催化作用。这些发现导致该化学范围的显着扩展,现在允许偶联各种环状胺、无环仲胺和伯胺。此外,我们还证明了这些新条件允许使用酰胺亲核试剂,除了胍和脲。
更新日期:2021-09-03
中文翻译:

Pd 催化烯烃二胺化反应与 O-苯甲酰羟胺亲电子试剂:支持 Pd(II/IV) 催化循环的证据、2,4-戊二酮衍生物作为配体的作用以及扩大的底物范围
本文描述了对作为 N 中心亲电试剂的N-烯丙基胍或脲与O-苯甲酰羟胺衍生物之间 Pd 催化的烯烃二化反应的持续研究。这些转化以中等至良好的产率产生了带有二烷基氨基甲基的环状胍和脲。我们描述了新的机制实验,这些实验导致了修正的机制假设,该假设涉及将亲电试剂关键的氧化添加到 Pd II复合物中,然后从 Pd IV中还原消除形成烷基碳-氮键。此外,我们证明了 acac,而不是磷化氢,是钯的关键配体。此外,带有取代芳基的简单 acac 衍生物在催化反应中的性能优于 acac,而膦在许多情况下会抑制催化作用。这些发现导致该化学范围的显着扩展,现在允许偶联各种环状胺、无环仲胺和伯胺。此外,我们还证明了这些新条件允许使用酰胺亲核试剂,除了胍和脲。



























 京公网安备 11010802027423号
京公网安备 11010802027423号