当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis of benzobisimidazole and bibenzimidazole-based bisnitriles as potential precursors for DNA minor groove binders
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-08-04 , DOI: 10.1002/jhet.4353 Abdelbasset A. Farahat 1, 2 , Satori Iwamoto 1 , Michael Roche 1 , David W. Boykin 3
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-08-04 , DOI: 10.1002/jhet.4353 Abdelbasset A. Farahat 1, 2 , Satori Iwamoto 1 , Michael Roche 1 , David W. Boykin 3
Affiliation
|
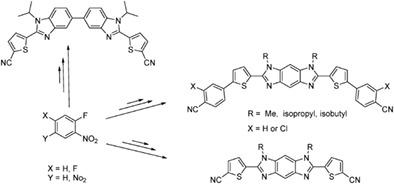
The synthesis of bisnitrile derivatives of benzobisimidazole and bibenzimidazole in a good yield is described in detail for the first time. Nucleophilic substitution of 1,5-difluoro-2,4-dinitrobenzene using different amines produced the intermediate diamines that were reduced using sodium borohydride/Pd(C) to produce the tetramines. These tetramines were allowed to couple with different aldehydes to produce the final benzimidazoles. In a different investigation, these bisnitrile derivatives will be used to make benzimidazole diamidines that could be used as potential mixed sequence minor groove binders.
中文翻译:

苯并双咪唑和双苯并咪唑基双腈作为 DNA 小沟结合剂的潜在前体的简便合成
首次详细描述了苯并双咪唑和联苯并咪唑的双腈衍生物的高产率合成。使用不同的胺对 1,5-二氟-2,4-二硝基苯进行亲核取代产生中间体二胺,使用硼氢化钠/Pd(C) 将其还原以产生四胺。允许这些四胺与不同的醛偶联以产生最终的苯并咪唑。在另一项研究中,这些双腈衍生物将用于制造苯并咪唑二脒,可用作潜在的混合序列小沟结合剂。
更新日期:2021-08-04
中文翻译:

苯并双咪唑和双苯并咪唑基双腈作为 DNA 小沟结合剂的潜在前体的简便合成
首次详细描述了苯并双咪唑和联苯并咪唑的双腈衍生物的高产率合成。使用不同的胺对 1,5-二氟-2,4-二硝基苯进行亲核取代产生中间体二胺,使用硼氢化钠/Pd(C) 将其还原以产生四胺。允许这些四胺与不同的醛偶联以产生最终的苯并咪唑。在另一项研究中,这些双腈衍生物将用于制造苯并咪唑二脒,可用作潜在的混合序列小沟结合剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号