当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and spectrophotometric investigation of two unnatural amino-acid altered chromophores in the superfolder green fluorescent protein
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-08-04 , DOI: 10.1107/s2059798321006525 Gregory M Olenginski 1 , Juliana Piacentini 1 , Darcy R Harris 1 , Nicolette A Runko 1 , Brianna M Papoutsis 1 , Jordan R Alter 1 , Kenneth R Hess 1 , Scott H Brewer 1 , Christine M Phillips-Piro 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-08-04 , DOI: 10.1107/s2059798321006525 Gregory M Olenginski 1 , Juliana Piacentini 1 , Darcy R Harris 1 , Nicolette A Runko 1 , Brianna M Papoutsis 1 , Jordan R Alter 1 , Kenneth R Hess 1 , Scott H Brewer 1 , Christine M Phillips-Piro 1
Affiliation
|
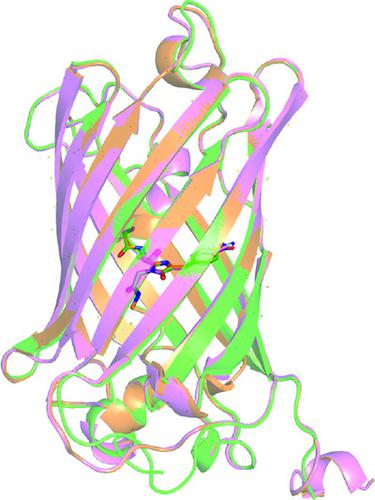
The spectrophotometric properties of the green fluorescent protein (GFP) result from the post-translationally cyclized chromophore composed of three amino acids including a tyrosine at the center of the β-barrel protein. Altering the amino acids in the chromophore or the nearby region has resulted in numerous GFP variants with differing photophysical properties. To further examine the effect of small atomic changes in the chromophore on the structure and photophysical properties of GFP, the hydroxyl group of the chromophore tyrosine was replaced with a nitro or a cyano group. The structures and spectrophotometric properties of these superfolder GFP (sfGFP) variants with the unnatural amino acids (UAAs) 4-nitro-l-phenylalanine or 4-cyano-l-phenylalanine were explored. Notably, the characteristic 487 nm absorbance band of wild-type (wt) sfGFP is absent in both unnatural amino-acid-containing protein constructs (Tyr66pNO2Phe-sfGFP and Tyr66pCNPhe-sfGFP). Consequently, neither Tyr66pNO2Phe-sfGFP nor Tyr66pCNPhe-sfGFP exhibited the characteristic emission of wt sfGFP centered at 511 nm when excited at 487 nm. Tyr66pNO2Phe-sfGFP appeared orange due to an absorbance band centered at 406 nm that was not present in wt sfGFP, while Tyr66pCNPhe-sfGFP appeared colorless with an absorbance band centered at 365 nm. Mass spectrometry and X-ray crystallography confirmed the presence of a fully formed chromophore and no significant structural changes in either of these UAA-containing protein constructs, signaling that the change in the observed photophysical properties of the proteins is the result of the presence of the UAA in the chromophore.
中文翻译:

超级文件夹绿色荧光蛋白中两种非天然氨基酸改变的发色团的结构和分光光度研究
绿色荧光蛋白 (GFP) 的分光光度特性源自翻译后环化发色团,该发色团由三个氨基酸组成,其中包括位于 β-桶蛋白中心的酪氨酸。改变发色团或附近区域中的氨基酸导致了许多具有不同光物理特性的 GFP 变体。为了进一步研究发色团中微小原子变化对 GFP 结构和光物理性质的影响,将发色团酪氨酸的羟基替换为硝基或氰基。探索了这些带有非天然氨基酸 (UAA) 4-硝基-1-苯丙氨酸或 4-氰基-1-苯丙氨酸的超级文件夹 GFP (sfGFP) 变体的结构和分光光度特性。值得注意的是,野生型 (wt) sfGFP 的特征性 487 nm 吸光度带在两种非天然含氨基酸蛋白质构建体(Tyr66pNO 2 Phe-sfGFP 和 Tyr66pCNPhe-sfGFP)中均不存在。因此,当在 487 nm 激发时,Tyr66pNO 2 Phe-sfGFP 和 Tyr66pCNPhe-sfGFP 均未表现出以 511 nm 为中心的 wt sfGFP 特征发射。Tyr66pNO 2 Phe-sfGFP 由于以 406 nm 为中心的吸光带而呈现橙色,而 wt sfGFP 中不存在该吸光带,而 Tyr66pCNPhe-sfGFP 则呈现无色,以 365 nm 为中心的吸光带。质谱分析和 X 射线晶体学证实了这些含有 UAA 的蛋白质构建体中存在完全形成的发色团,并且没有显着的结构变化,这表明观察到的蛋白质光物理性质的变化是由于发色团中的 UAA。
更新日期:2021-08-04
中文翻译:

超级文件夹绿色荧光蛋白中两种非天然氨基酸改变的发色团的结构和分光光度研究
绿色荧光蛋白 (GFP) 的分光光度特性源自翻译后环化发色团,该发色团由三个氨基酸组成,其中包括位于 β-桶蛋白中心的酪氨酸。改变发色团或附近区域中的氨基酸导致了许多具有不同光物理特性的 GFP 变体。为了进一步研究发色团中微小原子变化对 GFP 结构和光物理性质的影响,将发色团酪氨酸的羟基替换为硝基或氰基。探索了这些带有非天然氨基酸 (UAA) 4-硝基-1-苯丙氨酸或 4-氰基-1-苯丙氨酸的超级文件夹 GFP (sfGFP) 变体的结构和分光光度特性。值得注意的是,野生型 (wt) sfGFP 的特征性 487 nm 吸光度带在两种非天然含氨基酸蛋白质构建体(Tyr66pNO 2 Phe-sfGFP 和 Tyr66pCNPhe-sfGFP)中均不存在。因此,当在 487 nm 激发时,Tyr66pNO 2 Phe-sfGFP 和 Tyr66pCNPhe-sfGFP 均未表现出以 511 nm 为中心的 wt sfGFP 特征发射。Tyr66pNO 2 Phe-sfGFP 由于以 406 nm 为中心的吸光带而呈现橙色,而 wt sfGFP 中不存在该吸光带,而 Tyr66pCNPhe-sfGFP 则呈现无色,以 365 nm 为中心的吸光带。质谱分析和 X 射线晶体学证实了这些含有 UAA 的蛋白质构建体中存在完全形成的发色团,并且没有显着的结构变化,这表明观察到的蛋白质光物理性质的变化是由于发色团中的 UAA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号