Synlett ( IF 1.7 ) Pub Date : 2021-08-02 , DOI: 10.1055/s-0037-1610780 Peter Langer 1, 2
|
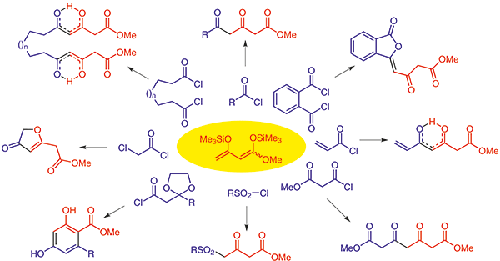
The reaction of 1,3-bis(silyloxy)-1,3-butadienes with functionalized and nonfunctionalized acid chlorides and bis(acid chlorides) gives rise to the formation of a great variety of 1,3,5-tri- and 1,3,5,7-tetracarbonyl compounds and cyclic products derived from them. Examples include functionalized and nonfunctionalized 3,5-dioxopentanoates, 3,5-dioxopimelates, 3-alkylidene-3H-isobenzofuran-1-ones, salicylates, 3(2H)furanones, and 3-oxo-4-sulfonylesters. Methylation and cyclopropanation reactions of 1,3,5-tri- and 1,3,5,7-tetracarbonyl compounds do not provide access to the expected permethylated and cyclopropanated polycarbonyl compounds. However, the latter could be prepared by stepwise protocols. The reaction of 1,3,5-tri- and 1,3,5,7-tetracarbonyl compounds with dinucleophiles resulted in cyclization reactions to give a variety of heterocyclic structures.
1 Introduction
2 Reactions of 1,3-Bis(silyloxy)-1,3-butadienes with Acid Chlorides
3 Reactions of 1,3,5-Tri- and 1,3,5,7-Tetracarbonyl Compounds
4 Conclusion
中文翻译:

1,3,5-三-和1,3,5,7-四羰基化合物的合成与反应
1,3-双(甲硅烷氧基)-1,3-丁二烯与官能化和非官能化的酰氯和双(酰氯)反应生成多种1,3,5-三-和1, 3,5,7-四羰基化合物及其衍生的环状产物。例子包括官能化和非官能化 3,5-dioxopentanoates、3,5-dioxopimelates、3-alkylidene-3 H -isobenzofuran -1-ones、水杨酸盐、3(2 H)呋喃酮和 3-oxo-4-磺酰酯。1,3,5-三-和 1,3,5,7-四羰基化合物的甲基化和环丙烷化反应无法获得预期的全甲基化和环丙烷化多羰基化合物。然而,后者可以通过逐步协议来准备。1,3,5-三-和1,3,5,7-四羰基化合物与双亲核试剂的反应导致环化反应,得到各种杂环结构。
1 简介
2 1,3-双(甲硅烷氧基)-1,3-丁二烯与酰氯的反应
3 1,3,5-三-和1,3,5,7-四羰基化合物的反应
4。结论











































 京公网安备 11010802027423号
京公网安备 11010802027423号