当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Environment and coordination of FeMo–co in the nitrogenase metallochaperone NafY
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-07-28 , DOI: 10.1039/d1cb00086a Aaron H Phillips 1 , Jose A Hernandez 2, 3 , Lucía Payá-Tormo 4 , Stefan Burén 4 , Bruno Cuevas-Zuviría 4 , Luis F Pacios 4 , Jeffrey G Pelton 5, 6 , David E Wemmer 5, 6, 7 , Luis M Rubio 4
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-07-28 , DOI: 10.1039/d1cb00086a Aaron H Phillips 1 , Jose A Hernandez 2, 3 , Lucía Payá-Tormo 4 , Stefan Burén 4 , Bruno Cuevas-Zuviría 4 , Luis F Pacios 4 , Jeffrey G Pelton 5, 6 , David E Wemmer 5, 6, 7 , Luis M Rubio 4
Affiliation
|
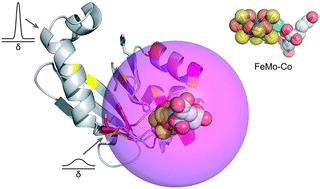
In nitrogenase biosynthesis, the iron-molybdenum cofactor (FeMo–co) is externally assembled at scaffold proteins and delivered to the NifDK nitrogenase component by the NafY metallochaperone. Here we have used nuclear magnetic resonance, molecular dynamics, and functional analysis to elucidate the environment and coordination of FeMo–co in NafY. H121 stands as the key FeMo–co ligand. Regions near FeMo–co diverge from H121 and include the η1, α1, α2 helical lobe and a narrow path between H121 and C196.
中文翻译:

FeMo-co 在固氮酶金属伴侣 NafY 中的环境和配位
在固氮酶生物合成中,铁钼辅因子 (FeMo-co) 在支架蛋白上外部组装,并通过 NafY 金属伴侣蛋白传递给 NifDK 固氮酶组分。在这里,我们使用核磁共振、分子动力学和功能分析来阐明 NafY 中 FeMo-co 的环境和协调。H 121是关键的 FeMo-co 配体。FeMo-co 附近的区域与 H 121 不同,包括 η1、α1、α2 螺旋瓣和 H 121和 C 196之间的狭窄路径。
更新日期:2021-08-03
中文翻译:

FeMo-co 在固氮酶金属伴侣 NafY 中的环境和配位
在固氮酶生物合成中,铁钼辅因子 (FeMo-co) 在支架蛋白上外部组装,并通过 NafY 金属伴侣蛋白传递给 NifDK 固氮酶组分。在这里,我们使用核磁共振、分子动力学和功能分析来阐明 NafY 中 FeMo-co 的环境和协调。H 121是关键的 FeMo-co 配体。FeMo-co 附近的区域与 H 121 不同,包括 η1、α1、α2 螺旋瓣和 H 121和 C 196之间的狭窄路径。









































 京公网安备 11010802027423号
京公网安备 11010802027423号