Developmental & Comparative Immunology ( IF 2.7 ) Pub Date : 2021-08-03 , DOI: 10.1016/j.dci.2021.104225 Jonathan P Rast 1 , Stefania D'Alessio 2 , Igor Kraev 3 , Sigrun Lange 2
|
Lampreys are a jawless vertebrate species belonging to an ancient vertebrate lineage that diverged from a common ancestor with humans ~500 million years ago. The sea lamprey (Petromyzon marinus) has a filter feeding ammocoete larval stage that metamorphoses into a parasitic adult, feeding both on teleost and elasmobranch fish. Lampreys are a valuable comparative model species for vertebrate immunity and physiology due to their unique phylogenetic position, unusual adaptive immune system, and physiological adaptions such as tolerance to salinity changes and urea.
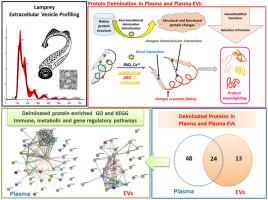
Peptidylarginine deiminases (PADs) are a phylogenetically conserved enzyme family which catalyses post-translational deimination/citrullination in target proteins, enabling proteins to gain new functions (moonlighting). The identification of deiminated protein targets in species across phylogeny may provide novel insights into post-translational regulation of physiological and pathophysiological processes. Extracellular vesicles (EVs) are membrane vesicles released from cells that carry cargos of small molecules and proteins for cellular communication, involved in both normal and pathological processes. The current study identified deimination signatures in proteins of both total plasma and plasma-EVs in sea lamprey and furthermore reports the first characterisation of plasma-EVs in lamprey. EVs were poly-dispersed in the size range of 40–500 nm, similar to what is observed in other taxa, positive for CD63 and Flotillin-1. Plasma-EV morphology was confirmed by transmission electron microscopy. Assessment of deimination/citrullination signatures in lamprey plasma and plasma-EVs, revealed 72 deimination target proteins involved in immunity, metabolism and gene regulation in whole plasma, and 37 target proteins in EVs, whereof 24 were shared targets. Furthermore, the presence of deiminated histone H3, indicative of gene-regulatory mechanisms and also a marker of neutrophil extracellular trap formation (NETosis), was confirmed in lamprey plasma. Functional protein network analysis revealed some differences in KEGG and GO pathways of deiminated proteins in whole plasma compared with plasma-EVs. For example, while common STRING network clusters in plasma and plasma-EVs included Peptide chain elongation, Viral mRNA translation, Glycolysis and gluconeogenesis, STRING network clusters specific for EVs only included: Cellular response to heat stress, Muscle protein and striated muscle thin filament, Nucleosome, Protein processing in endoplasmic reticulum, Nucleosome and histone deacetylase complex. STRING network clusters specific for plasma were: Adipokinetic hormone receptor activity, Fibrinogen alpha/beta chain family, peptidase S1A, Glutathione synthesis and recycling-arginine, Fructose 1,6-bisphosphate metabolic process, Carbon metabolism and lactate dehydrogenase activity, Post-translational protein phosphorylation, Regulation of insulin-like growth factor transport and clotting cascade. Overall, for the EV citrullinome, five STRING network clusters, 10 KEGG pathways, 15 molecular GO pathways and 29 Reactome pathways were identified, compared with nine STRING network clusters, six KEGG pathways, two Molecular GO pathways and one Reactome pathway specific for whole plasma; while further pathways were shared. The reported findings indicate that major pathways relevant for immunity and metabolism are targets of deimination in lamprey plasma and plasma-EVs, with some differences, and may help elucidating roles for the conserved PAD enzyme family in regulation of immune and metabolic function throughout phylogeny.
中文翻译:

海七鳃鳗(Petromyzon marinus)血浆和血浆细胞外囊泡中的翻译后蛋白质脱亚胺特征
七鳃鳗是一种无颌脊椎动物,属于古老的脊椎动物谱系,大约在 5 亿年前与人类的共同祖先分道扬镳。海七鳃鳗 ( Petromyzon marinus ) 有一个滤食性的幼虫阶段,该阶段变态成寄生成虫,以硬骨鱼和软鳃鱼为食。由于其独特的系统发育位置、不寻常的适应性免疫系统以及对盐度变化和尿素的耐受性等生理适应性,七鳃鳗是脊椎动物免疫和生理学的一种有价值的比较模型物种。
肽精氨酸脱亚胺酶 (PAD) 是一个系统发育保守的酶家族,可催化靶蛋白中的翻译后脱亚胺/瓜氨酸化,使蛋白质获得新功能(兼职)。跨系统发育的物种中脱亚胺蛋白靶标的鉴定可能为生理和病理生理过程的翻译后调控提供新的见解。细胞外囊泡 (EVs) 是从细胞释放的膜囊泡,其携带用于细胞通讯的小分子和蛋白质货物,参与正常和病理过程。目前的研究确定了海七鳃鳗中总血浆和血浆-EVs 的蛋白质中的脱亚胺特征,并进一步报告了七鳃鳗中血浆-EVs 的第一个特征。EV 多分散在 40-500 nm 的尺寸范围内,与在其他分类群中观察到的相似,CD63 和 Flotillin-1 呈阳性。通过透射电子显微镜确认等离子体-EV形态。对七鳃鳗血浆和血浆-EVs中脱亚胺/瓜氨酸化特征的评估揭示了全血浆中72个参与免疫、代谢和基因调控的脱亚胺靶蛋白,以及EVs中的37个靶蛋白,其中24个是共享靶蛋白。此外,在七鳃鳗血浆中证实了脱亚胺组蛋白 H3 的存在,表明基因调控机制,也是中性粒细胞胞外陷阱形成 (NETosis) 的标志物。功能蛋白网络分析显示,与血浆-EV相比,全血浆中脱亚胺蛋白的 KEGG 和 GO 通路存在一些差异。例如,虽然血浆和血浆 EV 中常见的 STRING 网络簇包括肽链延长、病毒 mRNA 翻译、糖酵解和糖异生,但 EV 特有的 STRING 网络簇仅包括:细胞对热应激的反应、肌肉蛋白和横纹肌细丝、核小体、蛋白质在内质网、核小体和组蛋白脱乙酰酶复合物中的加工。血浆特有的 STRING 网络簇包括:脂肪动力激素受体活性、纤维蛋白原 α/β 链家族、肽酶 S1A、谷胱甘肽合成和再循环-精氨酸、果糖 1,6-二磷酸代谢过程、碳代谢和乳酸脱氢酶活性、翻译后蛋白磷酸化,胰岛素样生长因子转运和凝血级联的调节。总体而言,对于 EV 瓜氨酸组,鉴定出 5 个 STRING 网络簇、10 个 KEGG 通路、15 个分子 GO 通路和 29 个 Reactome 通路,与 9 个 STRING 网络簇、6 个 KEGG 通路、2 个分子 GO 通路和 1 个全血浆特异性反应组通路相比;同时分享了进一步的途径。报告的研究结果表明,与免疫和代谢相关的主要途径是七鳃鳗血浆和血浆-EV 中脱亚胺的目标,但存在一些差异,这可能有助于阐明保守的 PAD 酶家族在整个系统发育过程中调节免疫和代谢功能的作用。同时分享了进一步的途径。报告的研究结果表明,与免疫和代谢相关的主要途径是七鳃鳗血浆和血浆-EV 中脱亚胺的目标,但存在一些差异,这可能有助于阐明保守的 PAD 酶家族在整个系统发育过程中调节免疫和代谢功能的作用。同时分享了进一步的途径。报告的研究结果表明,与免疫和代谢相关的主要途径是七鳃鳗血浆和血浆-EV 中脱亚胺的目标,但存在一些差异,这可能有助于阐明保守的 PAD 酶家族在整个系统发育过程中调节免疫和代谢功能的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号