Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-08-03 , DOI: 10.1016/j.jcou.2021.101662 Jie Zhao 1, 2 , Shuai Deng 1, 2 , Li Zhao 1 , Xiangzhou Yuan 3 , Bin Wang 4 , Lijin Chen 1, 2 , Kailong Wu 1, 2
|
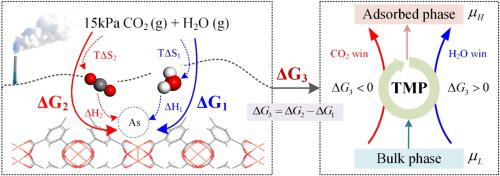
The presence of water vapor in post-combustion gas streams is a challenging technical issue that limits the utilization of CO2 adsorbents. Understanding the physical and chemical interaction mechanisms between adsorbents and adsorbates is the key to effectively deal with the competitive adsorption of CO2 and H2O. In this paper, a thermodynamic molecular pump (TMP) is proposed to resolve the contradictions between the promotion and impedance of CO2 adsorption by H2O. Specifically, the TMP model was proposed for quantitative analyses on a molecular scale for the first time. The equilibrium adsorption isotherms and adsorption enthalpies for CO2 and H2O at 298–388 K were obtained by grand canonical Monte Carlo (GCMC) simulations, while the adsorption entropies were obtained by density functional theory (DFT). Furthermore, the Gibbs free adsorption energy was then obtained by considering the effects of temperature and adsorption sites. The TMP-based analysis showed that the Gibbs free adsorption energy of H2O was a significant factor that influenced the CO2 adsorption result due to its contribution to the total driving energy. For CuBTC (BTC: benzene-1,3,5-tricarboxylate), the CO2 adsorption entropy provided a greater driving force to adsorb CO2 preferentially at 348–388 K. For zeolite AFI, the adsorption enthalpy was always the largest driving force. The results show that the adsorption temperature had a strong influence on zeolites. At 320–360 K, the zeolites showed better CO2/H2O adsorption selectivity than metal–organic frameworks (MOFs) due to their sensitivity to the thermal driving forces, as predicted by the TMP model. This is especially significant for the potential application of zeolites in temperature swing cycles driven by low- and medium-grade energy. The TMP model can provide guidance for screening CO2 adsorbents in the presence of H2O.
中文翻译:

H2O 对 CO2 吸附捕获的协同和竞争作用:基于分子动力学模拟的机理解释
燃烧后气流中水蒸气的存在是一个具有挑战性的技术问题,限制了 CO 2吸附剂的利用。了解吸附剂与被吸附物之间的物理化学相互作用机制是有效应对 CO 2和 H 2 O竞争吸附的关键。本文提出了一种热力学分子泵(TMP)来解决促进和吸附之间的矛盾。H 2 O吸附CO 2 的阻抗。具体而言,首次提出了 TMP 模型用于分子尺度上的定量分析。CO 2和H 2的平衡吸附等温线和吸附焓298-388 K 下的 O 是通过正则蒙特卡罗(GCMC)模拟获得的,而吸附熵是通过密度泛函理论(DFT)获得的。此外,通过考虑温度和吸附位点的影响,获得吉布斯自由吸附能。基于 TMP 的分析表明,H 2 O的吉布斯自由吸附能是影响 CO 2吸附结果的重要因素,因为它对总驱动能有贡献。对于CuBTC(BTC:benzo-1,3,5-tricarboxylate),CO 2吸附熵为吸附CO 2提供了更大的驱动力优先在 348-388 K。对于沸石 AFI,吸附焓始终是最大的驱动力。结果表明吸附温度对沸石的影响很大。正如 TMP 模型预测的那样,由于沸石对热驱动力的敏感性,在 320-360 K 下,沸石显示出比金属有机骨架 (MOF)更好的 CO 2 /H 2 O 吸附选择性。这对于沸石在中低档能源驱动的温度摆动循环中的潜在应用尤为重要。TMP 模型可以为在 H 2 O存在下筛选 CO 2吸附剂提供指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号