当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diverse functionalization of aryl halides mediated by bis(phenylsulfonyl)methane
Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-03 , DOI: 10.1016/j.tet.2021.132371 Ping Pan 1 , Lei Chen 1 , Xue-jing Zhang 1 , Ming Yan 1
中文翻译:

双(苯磺酰基)甲烷介导的芳基卤化物的多样化功能化
更新日期:2021-08-27
Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-03 , DOI: 10.1016/j.tet.2021.132371 Ping Pan 1 , Lei Chen 1 , Xue-jing Zhang 1 , Ming Yan 1
Affiliation
|
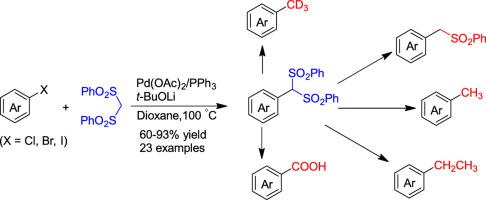
A palladium-catalyzed coupling reaction of bis(phenylsulfonyl)methane and aryl halides was developed. A variety of bis(phenylsulfonyl)methyl arenes were prepared in good yields. The transformations of bis(phenylsulfonyl)methyl to methyl, trideuteriomethyl, ethyl, carboxyl, and other functional groups were demonstrated. The results provided a new approach to diverse functionalization of aryl halides.
中文翻译:

双(苯磺酰基)甲烷介导的芳基卤化物的多样化功能化
开发了钯催化的双(苯磺酰基)甲烷和芳基卤化物的偶联反应。以良好的收率制备了多种双(苯磺酰基)甲基芳烃。证明了双(苯磺酰基)甲基向甲基、三氘代甲基、乙基、羧基和其他官能团的转化。该结果为芳基卤化物的多种功能化提供了一种新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号