Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling Sporadic Alzheimer's Disease in Human Brain Organoids under Serum Exposure
Advanced Science ( IF 14.3 ) Pub Date : 2021-08-02 , DOI: 10.1002/advs.202101462 Xianwei Chen 1 , Guoqiang Sun 1 , E Tian 1 , Mingzi Zhang 1 , Hayk Davtyan 2 , Thomas G Beach 3 , Eric M Reiman 4 , Mathew Blurton-Jones 5 , David M Holtzman 6 , Yanhong Shi 1
Advanced Science ( IF 14.3 ) Pub Date : 2021-08-02 , DOI: 10.1002/advs.202101462 Xianwei Chen 1 , Guoqiang Sun 1 , E Tian 1 , Mingzi Zhang 1 , Hayk Davtyan 2 , Thomas G Beach 3 , Eric M Reiman 4 , Mathew Blurton-Jones 5 , David M Holtzman 6 , Yanhong Shi 1
Affiliation
|
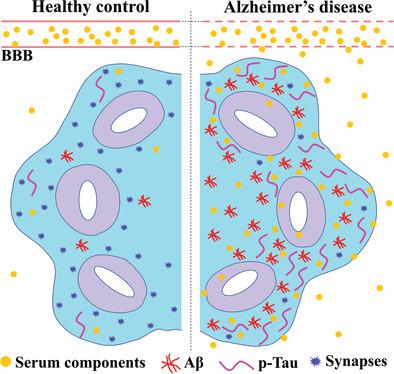
Alzheimer's disease (AD) is a progressive neurodegenerative disease with no cure. Huge efforts have been made to develop anti-AD drugs in the past decades. However, all drug development programs for disease-modifying therapies have failed. Possible reasons for the high failure rate include incomplete understanding of complex pathophysiology of AD, especially sporadic AD (sAD), and species difference between humans and animal models used in preclinical studies. In this study, sAD is modeled using human induced pluripotent stem cell (hiPSC)-derived 3D brain organoids. Because the blood–brain barrier (BBB) leakage is a well-known risk factor for AD, brain organoids are exposed to human serum to mimic the serum exposure consequence of BBB breakdown in AD patient brains. The serum-exposed brain organoids are able to recapitulate AD-like pathologies, including increased amyloid beta (Aβ) aggregates and phosphorylated microtubule-associated tau protein (p-Tau) level, synaptic loss, and impaired neural network. Serum exposure increases Aβ and p-Tau levels through inducing beta-secretase 1 (BACE) and glycogen synthase kinase-3 alpha / beta (GSK3α/β) levels, respectively. In addition, single-cell transcriptomic analysis of brain organoids reveals that serum exposure reduced synaptic function in both neurons and astrocytes and induced immune response in astrocytes. The human brain organoid-based sAD model established in this study can provide a powerful platform for both mechanistic study and therapeutic development in the future.
中文翻译:

血清暴露下人脑类器官中散发性阿尔茨海默病的建模
阿尔茨海默病(AD)是一种进行性神经退行性疾病,无法治愈。过去几十年来,人们为开发抗AD药物付出了巨大的努力。然而,所有疾病缓解疗法的药物开发计划都失败了。高失败率的可能原因包括对 AD 复杂病理生理学的不完全理解,尤其是散发性 AD (sAD),以及临床前研究中使用的人类和动物模型之间的物种差异。在这项研究中,使用人类诱导多能干细胞 (hiPSC) 衍生的 3D 脑类器官对 sAD 进行建模。由于血脑屏障 (BBB) 渗漏是 AD 的一个众所周知的危险因素,因此将脑类器官暴露于人血清中,以模拟 AD 患者大脑中 BBB 破裂的血清暴露后果。暴露于血清的脑类器官能够重现 AD 样病理,包括β淀粉样蛋白 (Aβ )聚集物增加和磷酸化微管相关 tau 蛋白 (p-Tau) 水平、突触损失和神经网络受损。血清暴露分别通过诱导 β 分泌酶 1 (BACE) 和糖原合成酶激酶 3 α/β (GSK3 α/β ) 水平来增加 A β和 p - Tau水平。此外,脑类器官的单细胞转录组分析表明,血清暴露会降低神经元和星形胶质细胞的突触功能,并诱导星形胶质细胞的免疫反应。本研究建立的基于人脑类器官的sAD模型可以为未来的机制研究和治疗开发提供强大的平台。
更新日期:2021-09-22
中文翻译:

血清暴露下人脑类器官中散发性阿尔茨海默病的建模
阿尔茨海默病(AD)是一种进行性神经退行性疾病,无法治愈。过去几十年来,人们为开发抗AD药物付出了巨大的努力。然而,所有疾病缓解疗法的药物开发计划都失败了。高失败率的可能原因包括对 AD 复杂病理生理学的不完全理解,尤其是散发性 AD (sAD),以及临床前研究中使用的人类和动物模型之间的物种差异。在这项研究中,使用人类诱导多能干细胞 (hiPSC) 衍生的 3D 脑类器官对 sAD 进行建模。由于血脑屏障 (BBB) 渗漏是 AD 的一个众所周知的危险因素,因此将脑类器官暴露于人血清中,以模拟 AD 患者大脑中 BBB 破裂的血清暴露后果。暴露于血清的脑类器官能够重现 AD 样病理,包括β淀粉样蛋白 (Aβ )聚集物增加和磷酸化微管相关 tau 蛋白 (p-Tau) 水平、突触损失和神经网络受损。血清暴露分别通过诱导 β 分泌酶 1 (BACE) 和糖原合成酶激酶 3 α/β (GSK3 α/β ) 水平来增加 A β和 p - Tau水平。此外,脑类器官的单细胞转录组分析表明,血清暴露会降低神经元和星形胶质细胞的突触功能,并诱导星形胶质细胞的免疫反应。本研究建立的基于人脑类器官的sAD模型可以为未来的机制研究和治疗开发提供强大的平台。









































 京公网安备 11010802027423号
京公网安备 11010802027423号