当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Dynamics Perturbations in Ubiquitin Adsorbed or Entrapped in Silica Materials Are Related to Disparate Surface Chemistries Resolved by Solid-State NMR Spectroscopy
Biomacromolecules ( IF 5.5 ) Pub Date : 2021-08-01 , DOI: 10.1021/acs.biomac.1c00495 Nurit Adiram-Filiba 1 , Eli Ohaion 1 , Gilit Verner 1 , Avital Schremer 1 , Merav Nadav-Tsubery 1 , Tammy Lublin-Tennenbaum 1 , Keren Keinan-Adamsky 1 , Massimo Lucci 2 , Claudio Luchinat 2 , Enrico Ravera 2 , Gil Goobes 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2021-08-01 , DOI: 10.1021/acs.biomac.1c00495 Nurit Adiram-Filiba 1 , Eli Ohaion 1 , Gilit Verner 1 , Avital Schremer 1 , Merav Nadav-Tsubery 1 , Tammy Lublin-Tennenbaum 1 , Keren Keinan-Adamsky 1 , Massimo Lucci 2 , Claudio Luchinat 2 , Enrico Ravera 2 , Gil Goobes 1
Affiliation
|
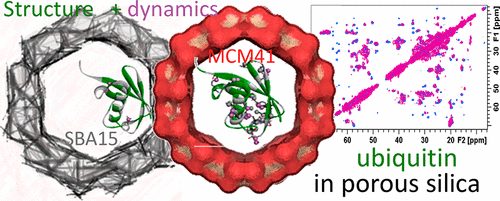
Protein immobilization on material surfaces is emerging as a powerful tool in the design of devices and active materials for biomedical and pharmaceutical applications as well as for catalysis. Preservation of the protein’s biological functionality is crucial to the design process and is dependent on the ability to maintain its structural and dynamical integrity while removed from the natural surroundings. The scientific techniques to validate the structure of immobilized proteins are scarce and usually provide limited information as a result of poor resolution. In this work, we benchmarked the ability of standard solid-state NMR techniques to resolve the effects of binding to dissimilar silica materials on a model protein. In particular, the interactions between ubiquitin and the surfaces of MCM41, SBA15, and silica formed in situ were tested for their influence on the structure and dynamics of the protein. It is shown that the protein’s globular fold in the free state is only slightly perturbed in the three silica materials. Local motions on a residue level that are quenched by immobilization or, conversely, that arise from the process are also detailed. NMR measurements show that these perturbations are unique to each silica material and can serve as reporters of the characteristic surface chemistry.
中文翻译:

吸附或包埋在二氧化硅材料中的泛素的结构和动力学扰动与固态核磁共振光谱解析的不同表面化学性质有关
材料表面的蛋白质固定正在成为设计用于生物医学和制药应用以及催化的装置和活性材料的强大工具。保留蛋白质的生物功能对设计过程至关重要,并且取决于在远离自然环境的同时保持其结构和动态完整性的能力。用于验证固定蛋白结构的科学技术很少,而且由于分辨率低,通常提供的信息有限。在这项工作中,我们对标准固态 NMR 技术解决与不同二氧化硅材料结合对模型蛋白质的影响的能力进行了基准测试。特别是泛素与 MCM41、SBA15、测试了原位形成的二氧化硅和二氧化硅对蛋白质结构和动力学的影响。结果表明,在三种二氧化硅材料中,游离状态下蛋白质的球状折叠仅受到轻微干扰。还详细介绍了通过固定化或相反地由该过程引起的残留物水平上的局部运动。核磁共振测量表明,这些扰动对每种二氧化硅材料都是独一无二的,可以作为特征表面化学的报告者。
更新日期:2021-09-13
中文翻译:

吸附或包埋在二氧化硅材料中的泛素的结构和动力学扰动与固态核磁共振光谱解析的不同表面化学性质有关
材料表面的蛋白质固定正在成为设计用于生物医学和制药应用以及催化的装置和活性材料的强大工具。保留蛋白质的生物功能对设计过程至关重要,并且取决于在远离自然环境的同时保持其结构和动态完整性的能力。用于验证固定蛋白结构的科学技术很少,而且由于分辨率低,通常提供的信息有限。在这项工作中,我们对标准固态 NMR 技术解决与不同二氧化硅材料结合对模型蛋白质的影响的能力进行了基准测试。特别是泛素与 MCM41、SBA15、测试了原位形成的二氧化硅和二氧化硅对蛋白质结构和动力学的影响。结果表明,在三种二氧化硅材料中,游离状态下蛋白质的球状折叠仅受到轻微干扰。还详细介绍了通过固定化或相反地由该过程引起的残留物水平上的局部运动。核磁共振测量表明,这些扰动对每种二氧化硅材料都是独一无二的,可以作为特征表面化学的报告者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号