当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Slow-Onset, Potent Inhibition of Mandelate Racemase by 2-Formylphenylboronic Acid. An Unexpected Adduct Clasps the Catalytic Machinery
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-02 , DOI: 10.1021/acs.biochem.1c00374 Colin D Douglas 1 , Lia Grandinetti 2 , Nicole M Easton 1 , Oliver P Kuehm 1 , Joshua A Hayden 1 , Meghan C Hamilton 1 , Martin St Maurice 2 , Stephen L Bearne 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-02 , DOI: 10.1021/acs.biochem.1c00374 Colin D Douglas 1 , Lia Grandinetti 2 , Nicole M Easton 1 , Oliver P Kuehm 1 , Joshua A Hayden 1 , Meghan C Hamilton 1 , Martin St Maurice 2 , Stephen L Bearne 1, 3
Affiliation
|
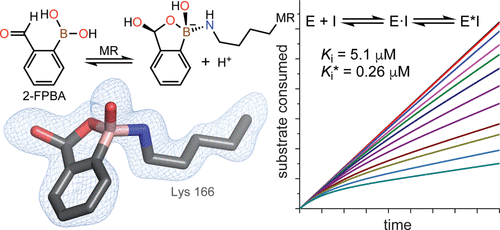
o-Carbonyl arylboronic acids such as 2-formylphenylboronic acid (2-FPBA) are employed in biocompatible conjugation reactions with the resulting iminoboronate adduct stabilized by an intramolecular N–B interaction. However, few studies have utilized these reagents as active site-directed enzyme inhibitors. We show that 2-FPBA is a potent reversible, slow-onset inhibitor of mandelate racemase (MR), an enzyme that has served as a valuable paradigm for understanding enzyme-catalyzed abstraction of an α-proton from a carbon acid substrate with a high pKa. Kinetic analysis of the progress curves for the slow onset of inhibition of wild-type MR using a two-step kinetic mechanism gave Ki and Ki* values of 5.1 ± 1.8 and 0.26 ± 0.08 μM, respectively. Hence, wild-type MR binds 2-FPBA with an affinity that exceeds that for the substrate by ∼3000-fold. K164R MR was inhibited by 2-FPBA, while K166R MR was not inhibited, indicating that Lys 166 was essential for inhibition. Unexpectedly, mass spectrometric analysis of the NaCNBH3-treated enzyme–inhibitor complex did not yield evidence of an iminoboronate adduct. 11B nuclear magnetic resonance spectroscopy of the MR·2-FPBA complex indicated that the boron atom was sp3-hybridized (δ 6.0), consistent with dative bond formation. Surprisingly, X-ray crystallography revealed the formation of an Nζ–B dative bond between Lys 166 and 2-FPBA with intramolecular cyclization to form a benzoxaborole, rather than the expected iminoboronate. Thus, when o-carbonyl arylboronic acid reagents are employed to modify proteins, the structure of the resulting product depends on the protein architecture at the site of modification.
中文翻译:

2-甲酰基苯基硼酸对扁桃酸消旋酶具有缓慢起效、强效的抑制作用。意外的加合物束缚了催化机制
邻羰基芳基硼酸,例如 2-甲酰基苯基硼酸 (2-FPBA),可用于生物相容性缀合反应,并通过分子内 N-B 相互作用稳定生成亚氨基硼酸酯加合物。然而,很少有研究利用这些试剂作为活性位点定向酶抑制剂。我们证明 2-FPBA 是一种有效的可逆、缓慢起效的扁桃酸消旋酶 (MR) 抑制剂,这种酶已成为理解酶催化从具有高催化活性的碳酸底物中提取 α-质子的有价值的范例。 pKa 。使用两步动力学机制对野生型 MR 抑制缓慢起效的进展曲线进行动力学分析,得出K i和K i * 值分别为 5.1 ± 1.8 和 0.26 ± 0.08 μM。因此,野生型 MR 结合 2-FPBA 的亲和力比底物的亲和力高约 3000 倍。 K164R MR 被 2-FPBA 抑制,而 K166R MR 未被抑制,表明 Lys 166 对于抑制至关重要。出乎意料的是,NaCNBH 3处理的酶抑制剂复合物的质谱分析没有产生亚氨基硼酸盐加合物的证据。 MR·2-FPBA配合物的11 B核磁共振波谱表明硼原子发生sp 3杂化(δ 6.0),这与配位键的形成一致。令人惊讶的是,X 射线晶体学揭示了 Lys 166 和 2-FPBA 之间形成了 N z -B 配位键,并进行分子内环化,形成苯并氧硼杂环,而不是预期的亚氨基硼酸酯。 因此,当使用邻羰基芳基硼酸试剂修饰蛋白质时,所得产物的结构取决于修饰位点的蛋白质结构。
更新日期:2021-08-17
中文翻译:

2-甲酰基苯基硼酸对扁桃酸消旋酶具有缓慢起效、强效的抑制作用。意外的加合物束缚了催化机制
邻羰基芳基硼酸,例如 2-甲酰基苯基硼酸 (2-FPBA),可用于生物相容性缀合反应,并通过分子内 N-B 相互作用稳定生成亚氨基硼酸酯加合物。然而,很少有研究利用这些试剂作为活性位点定向酶抑制剂。我们证明 2-FPBA 是一种有效的可逆、缓慢起效的扁桃酸消旋酶 (MR) 抑制剂,这种酶已成为理解酶催化从具有高催化活性的碳酸底物中提取 α-质子的有价值的范例。 pKa 。使用两步动力学机制对野生型 MR 抑制缓慢起效的进展曲线进行动力学分析,得出K i和K i * 值分别为 5.1 ± 1.8 和 0.26 ± 0.08 μM。因此,野生型 MR 结合 2-FPBA 的亲和力比底物的亲和力高约 3000 倍。 K164R MR 被 2-FPBA 抑制,而 K166R MR 未被抑制,表明 Lys 166 对于抑制至关重要。出乎意料的是,NaCNBH 3处理的酶抑制剂复合物的质谱分析没有产生亚氨基硼酸盐加合物的证据。 MR·2-FPBA配合物的11 B核磁共振波谱表明硼原子发生sp 3杂化(δ 6.0),这与配位键的形成一致。令人惊讶的是,X 射线晶体学揭示了 Lys 166 和 2-FPBA 之间形成了 N z -B 配位键,并进行分子内环化,形成苯并氧硼杂环,而不是预期的亚氨基硼酸酯。 因此,当使用邻羰基芳基硼酸试剂修饰蛋白质时,所得产物的结构取决于修饰位点的蛋白质结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号