当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
What's in the BAGs? Intrinsic disorder angle of the multifunctionality of the members of a family of chaperone regulators
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-08-02 , DOI: 10.1002/jcb.30123 Liberato Marzullo 1, 2 , Maria C Turco 1, 2 , Vladimir N Uversky 3
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-08-02 , DOI: 10.1002/jcb.30123 Liberato Marzullo 1, 2 , Maria C Turco 1, 2 , Vladimir N Uversky 3
Affiliation
|
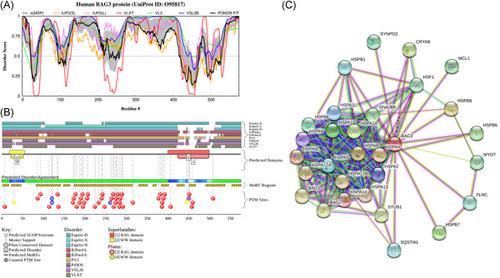
In humans, the family of Bcl-2 associated athanogene (BAG) proteins includes six members characterized by exceptional multifunctionality and engagement in the pathogenesis of various diseases. All of them are capable of interacting with a multitude of often unrelated binding partners. Such binding promiscuity and related functional and pathological multifacetedness cannot be explained or understood within the frames of the classical “one protein–one structure–one function” model, which also fails to explain the presence of multiple isoforms generated for BAG proteins by alternative splicing or alternative translation initiation and their extensive posttranslational modifications. However, all these mysteries can be solved by taking into account the intrinsic disorder phenomenon. In fact, high binding promiscuity and potential to participate in a broad spectrum of interactions with multiple binding partners, as well as a capability to be multifunctional and multipathogenic, are some of the characteristic features of intrinsically disordered proteins and intrinsically disordered protein regions. Such functional proteins or protein regions lacking unique tertiary structures constitute a cornerstone of the protein structure-function continuum concept. The aim of this paper is to provide an overview of the functional roles of human BAG proteins from the perspective of protein intrinsic disorder which will provide a means for understanding their binding promiscuity, multifunctionality, and relation to the pathogenesis of various diseases.
中文翻译:

袋子里有什么?伴侣调节器家族成员多功能性的内在障碍角度
在人类中,Bcl-2 相关的 athanogene (BAG) 蛋白家族包括六个成员,其特点是具有特殊的多功能性和参与各种疾病的发病机制。它们都能够与许多通常不相关的绑定伙伴进行交互。在经典的“一个蛋白质 - 一个结构 - 一个功能”模型的框架内无法解释或理解这种结合混杂和相关的功能和病理多方面性,该模型也无法解释通过选择性剪接或为 BAG 蛋白产生的多个同种型的存在。替代翻译起始及其广泛的翻译后修饰。然而,所有这些谜团都可以通过考虑内在的无序现象来解决。实际上,高结合杂乱性和参与与多个结合伙伴的广泛相互作用的潜力,以及多功能和多病原性的能力,是本质上无序的蛋白质和本质上无序的蛋白质区域的一些特征。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。以及具有多功能性和多病原性的能力,是本质上无序的蛋白质和本质上无序的蛋白质区域的一些特征。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。以及具有多功能性和多病原性的能力,是本质上无序的蛋白质和本质上无序的蛋白质区域的一些特征。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。
更新日期:2021-08-02
中文翻译:

袋子里有什么?伴侣调节器家族成员多功能性的内在障碍角度
在人类中,Bcl-2 相关的 athanogene (BAG) 蛋白家族包括六个成员,其特点是具有特殊的多功能性和参与各种疾病的发病机制。它们都能够与许多通常不相关的绑定伙伴进行交互。在经典的“一个蛋白质 - 一个结构 - 一个功能”模型的框架内无法解释或理解这种结合混杂和相关的功能和病理多方面性,该模型也无法解释通过选择性剪接或为 BAG 蛋白产生的多个同种型的存在。替代翻译起始及其广泛的翻译后修饰。然而,所有这些谜团都可以通过考虑内在的无序现象来解决。实际上,高结合杂乱性和参与与多个结合伙伴的广泛相互作用的潜力,以及多功能和多病原性的能力,是本质上无序的蛋白质和本质上无序的蛋白质区域的一些特征。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。以及具有多功能性和多病原性的能力,是本质上无序的蛋白质和本质上无序的蛋白质区域的一些特征。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。以及具有多功能性和多病原性的能力,是本质上无序的蛋白质和本质上无序的蛋白质区域的一些特征。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。这种缺乏独特三级结构的功能性蛋白质或蛋白质区域构成了蛋白质结构-功能连续统概念的基石。本文的目的是从蛋白质内在疾病的角度概述人类 BAG 蛋白的功能作用,这将为理解它们的结合混杂性、多功能性以及与各种疾病的发病机制的关系提供一种手段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号