当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Acinetobacter baumannii Autotransporter Adhesin Ata Recognizes Host Glycans as High-Affinity Receptors
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-08-02 , DOI: 10.1021/acsinfecdis.1c00021 Greg Tram 1 , Jessica Poole 1 , Felise G Adams 2 , Michael P Jennings 1 , Bart A Eijkelkamp 2 , John M Atack 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-08-02 , DOI: 10.1021/acsinfecdis.1c00021 Greg Tram 1 , Jessica Poole 1 , Felise G Adams 2 , Michael P Jennings 1 , Bart A Eijkelkamp 2 , John M Atack 1
Affiliation
|
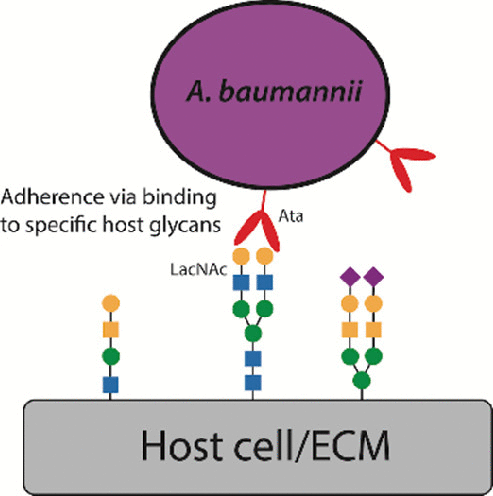
Acinetobacter baumannii is a significant opportunistic pathogen responsible for infections of the lung, blood, skin, urinary tract, and soft tissues, with some strains exhibiting almost complete resistance to commonly used antibiotics. This multidrug resistance, together with a dearth of new antibiotic development, mean novel methods of treatment and prevention are urgently needed. Although many A. baumannii factors required to colonize the host have been identified, little is known about the specific host molecules recognized by these factors. A. baumannii produces a trimeric autotransporter adhesin known as Ata that has been previously demonstrated to bind components of the host cell’s extracellular matrix, which are often heavily glycosylated. We hypothesized that Ata would exhibit lectin activity which would play a role in adherence to the host cell surface. Our biophysical analysis using glycan arrays and surface plasmon resonance demonstrated that Ata binds galactose, N-acetylglucosamine, and galactose (β1–3/4) N-acetylglucosamine with high-affinity. These structures are present on many of the proteins which were previously reported to be bound by Ata. We also demonstrated that the recognition of human plasma fibronectin by Ata requires this ability to bind glycans, as the interaction between Ata and fibronectin does not occur when fibronectin is deglycosylated. This strongly suggests a key role for Ata lectin activity during host adherence. This information will assist in directing the development of new and effective treatments to block host interactions using glycans and/or novel compounds in multidrug resistant A. baumannii infections.
中文翻译:

鲍曼不动杆菌自转运体粘附素 Ata 将宿主聚糖识别为高亲和力受体
鲍曼不动杆菌是一种重要的机会性病原体,可导致肺、血液、皮肤、泌尿道和软组织感染,某些菌株对常用抗生素几乎完全耐药。这种多药耐药性加上新抗生素开发的缺乏,意味着迫切需要新的治疗和预防方法。尽管已经确定了寄居宿主所需的许多鲍曼不动杆菌因子,但对这些因子识别的特定宿主分子知之甚少。鲍曼不动杆菌产生一种称为 Ata 的三聚体自转运粘附素,以前已证明它可以结合宿主细胞的细胞外基质的成分,这些成分通常是高度糖基化的。我们假设 Ata 会表现出凝集素活性,这将在粘附到宿主细胞表面方面发挥作用。我们使用聚糖阵列和表面等离子体共振的生物物理分析表明,Ata 结合半乳糖、N-乙酰氨基葡萄糖和半乳糖 (β1–3/4) N-乙酰氨基葡萄糖具有高亲和力。这些结构存在于许多先前报道为被 Ata 结合的蛋白质上。我们还证明了 Ata 对人血浆纤连蛋白的识别需要这种结合聚糖的能力,因为当纤连蛋白去糖基化时不会发生 Ata 和纤连蛋白之间的相互作用。这强烈暗示了 Ata 凝集素活性在宿主粘附过程中的关键作用。该信息将有助于指导开发新的有效治疗方法,以在多重耐药鲍曼不动杆菌感染中使用聚糖和/或新型化合物阻断宿主相互作用。
更新日期:2021-08-13
中文翻译:

鲍曼不动杆菌自转运体粘附素 Ata 将宿主聚糖识别为高亲和力受体
鲍曼不动杆菌是一种重要的机会性病原体,可导致肺、血液、皮肤、泌尿道和软组织感染,某些菌株对常用抗生素几乎完全耐药。这种多药耐药性加上新抗生素开发的缺乏,意味着迫切需要新的治疗和预防方法。尽管已经确定了寄居宿主所需的许多鲍曼不动杆菌因子,但对这些因子识别的特定宿主分子知之甚少。鲍曼不动杆菌产生一种称为 Ata 的三聚体自转运粘附素,以前已证明它可以结合宿主细胞的细胞外基质的成分,这些成分通常是高度糖基化的。我们假设 Ata 会表现出凝集素活性,这将在粘附到宿主细胞表面方面发挥作用。我们使用聚糖阵列和表面等离子体共振的生物物理分析表明,Ata 结合半乳糖、N-乙酰氨基葡萄糖和半乳糖 (β1–3/4) N-乙酰氨基葡萄糖具有高亲和力。这些结构存在于许多先前报道为被 Ata 结合的蛋白质上。我们还证明了 Ata 对人血浆纤连蛋白的识别需要这种结合聚糖的能力,因为当纤连蛋白去糖基化时不会发生 Ata 和纤连蛋白之间的相互作用。这强烈暗示了 Ata 凝集素活性在宿主粘附过程中的关键作用。该信息将有助于指导开发新的有效治疗方法,以在多重耐药鲍曼不动杆菌感染中使用聚糖和/或新型化合物阻断宿主相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号