The International Journal of Biochemistry & Cell Biology ( IF 3.4 ) Pub Date : 2021-07-31 , DOI: 10.1016/j.biocel.2021.106051 S Bindhya 1 , C Sidhanth 1 , S Krishnapriya 1 , Manoj Garg 2 , T S Ganesan 1
|
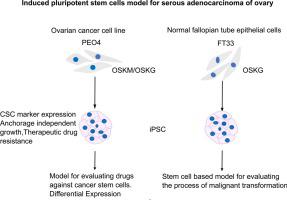
Ovarian cancer recurs despite advances in treatment and is due to drug resistance. The persistence of cancer stem cells (CSCs) is one of the causes. It has been challenging to maintain CSCs long term in culture from primary malignant cells. Reprogramming cancer cells into induced pluripotent stem cells (iPSCs) could be an approach to achieve this. An ovarian cancer cell line, PEO4, was initially reprogrammed into iPSCs using the classical four factors OCT4, SOX2, KLF4 and MYC (OSKM) using lentivirus transduction. The PEO4-OSKM-cells had all the hallmarks of iPSCs. As MYC is oncogenic, we have replaced it with GLIS1 and show that PEO4 cells could be transformed into iPSCs. The transfection efficiency was two-fold better with OCT4-SOX2-KLF4-GLIS1 (OSKG) with larger colonies. Further, normal fallopian tube epithelial cells were also transformed using OSKG into iPSCs. iPSCs expressed CSCs markers such as CD133, EPHA1, ALDH1A1 and LGR5 prominently and were more resistant to cisplatin and taxol as compared to parental PEO4 cells. PEO4-OSKM-iPSCs cells formed more colonies in a clonogenic assay as compared to PEO4-OSKG-iPSCs and parental cells. These results provide a first insight that both an ovarian cancer cell line and fallopian tube epithelial cells can be reprogrammed and GLIS1 can successfully replace MYC as a transcription factor. This in vitro model is useful for future experiments to understand the characteristics of CSCs in the pathogenesis of ovarian cancer.
中文翻译:

卵巢癌诱导多能干细胞模型的开发和体外表征
尽管治疗取得进展,卵巢癌仍会复发,这是由于耐药性。癌症干细胞 (CSC) 的持续存在是原因之一。在原代恶性细胞培养中长期维持 CSCs 一直具有挑战性。将癌细胞重编程为诱导多能干细胞 (iPSC) 可能是实现这一目标的一种方法。使用慢病毒转导,使用经典的四个因子OCT 4、SOX2、KLF4和MYC (OSKM)最初将卵巢癌细胞系 PEO4 重编程为 iPSC 。PEO4-OSKM 细胞具有 iPSC 的所有特征。由于MYC具有致癌性,我们已将其替换为GLIS1并表明 PEO4 细胞可以转化为 iPSC。使用具有较大菌落的 OCT4-SOX2-KLF4-GLIS1 (OSKG) 的转染效率提高两倍。此外,正常输卵管上皮细胞也使用 OSKG 转化为 iPSC。的iPSC表达的CSC标记物如CD133,EPHA1,ALDH1A1和LGR5突出并分别对顺铂和紫杉醇的抗性相比亲本PEO4细胞。与 PEO4-OSKG-iPSCs 和亲代细胞相比,PEO4-OSKM-iPSCs 细胞在克隆形成试验中形成了更多的集落。这些结果提供了第一个见解,即卵巢癌细胞系和输卵管上皮细胞都可以重新编程,并且GLIS1可以成功取代MYC作为转录因子。这个在体外模型是有用的未来的实验来了解卵巢癌的发病肿瘤干细胞的特性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号