Experimental Neurology ( IF 4.6 ) Pub Date : 2021-07-31 , DOI: 10.1016/j.expneurol.2021.113828 Kaiyi Zhu 1 , Xing Zhu 1 , Shenghui Sun 2 , Wei Yang 3 , Shiqi Liu 1 , Zhen Tang 4 , Rong Zhang 5 , Jian Li 2 , Tao Shen 2 , Mingyan Hei 1
|
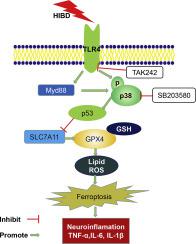
Inflammation and cell death play important roles in the pathogenesis of hypoxic-ischemic brain damage (HIBD). Toll-like receptor 4 (TLR4) triggers the activation of the inflammatory pathway. Ferroptosis, a newly identified type of regulated cell death, is implicated in various diseases involving neuronal injury. However, the role of ferroptosis in HIBD has not been elucidated. The objectives of this study were to explore the function and mechanism of TLR4 in neuronal ferroptosis in the context of HIBD. A neonatal rat model of hypoxia-ischemia (HI) and a cell model of oxygen-glucose deprivation (OGD) were employed. TAK-242, a TLR4-specific antagonist, was used to evaluate the effect of TLR4 on neuronal ferroptosis in vivo. A TAK-242 inhibitor and a p38 inhibitor (SB203580) were administered to HT22 hippocampal neurons to explore the association between TLR4 in inflammation and ferroptosis in vitro. The effects of TLR4 on ferroptosis were assessed by the Western blot, real-time PCR, immunofluorescence staining, cell viability and transmission electron microscopy (TEM) assays. HI insult significantly upregulated the TLR4, increased the p53 level, reduced the SLC7A11 and GPX4 levels, and caused mitochondrial damage, thereby inducing neuronal ferroptosis in the hippocampus. Inhibition of TLR4 inhibited the expression of ferroptosis-related proteins, decreased the expression of ferroptosis-related genes and the proinflammatory milieu, attenuated oxidative stress and mitochondrial injury and, finally, ameliorated the activation of hippocampal neuronal ferroptosis following HIBD. Consistent with the results of these in vivo experiments, TLR4 inhibition also attenuated OGD-induced ferroptosis by suppressing oxidative stress and p38MAPK signaling, ultimately increasing neuronal cell viability. Finally, the in vitro and in vivo results demonstrated that TAK-242 exerted neuroprotective and antiferroptotic effects by suppressing TLR4-p38 MAPK signaling. TLR4 activation induced neuronal ferroptosis following both HIBD and OGD. Inhibition of TLR4 attenuated oxidative stress-induced damage, decreased the activation of ferroptosis, and attenuated neuroinflammation following HIBD. In this study, we demonstrated that the inhibition of TLR4-p38 MAPK signaling modulates HIBD- or OGD-induced ferroptosis in neuronal cells and may play a novel role in brain homeostasis.
中文翻译:

抑制TLR4通过调节新生大鼠铁死亡防止海马缺氧缺血性损伤
炎症和细胞死亡在缺氧缺血性脑损伤 (HIBD) 的发病机制中起重要作用。Toll 样受体 4 (TLR4) 触发炎症通路的激活。Ferroptosis 是一种新发现的受调控的细胞死亡类型,与各种涉及神经元损伤的疾病有关。然而,铁死亡在 HIBD 中的作用尚未阐明。本研究的目的是探讨 TLR4 在 HIBD 背景下神经元铁死亡中的功能和机制。采用缺氧缺血(HI)的新生大鼠模型和氧-葡萄糖剥夺(OGD)的细胞模型。TAK-242 是一种 TLR4 特异性拮抗剂,用于评估 TLR4 对体内神经元铁死亡的影响。将 TAK-242 抑制剂和 p38 抑制剂 (SB203580) 施用于 HT22 海马神经元,以探索 TLR4 在炎症中与体外铁死亡之间的关联。TLR4 对铁死亡的影响通过蛋白质印迹、实时 PCR、免疫荧光染色、细胞活力和透射电子显微镜 (TEM) 测定进行评估。HI insult 显着上调 TLR4,增加 p53 水平,降低 SLC7A11 和 GPX4 水平,并引起线粒体损伤,从而诱导海马神经元铁死亡。抑制 TLR4 抑制铁死亡相关蛋白的表达,降低铁死亡相关基因和促炎环境的表达,减轻氧化应激和线粒体损伤,最后,改善了 HIBD 后海马神经元铁死亡的激活。与这些体内实验的结果一致,TLR4 抑制还通过抑制氧化应激和 p38MAPK 信号传导来减弱 OGD 诱导的铁死亡,最终增加神经元细胞的活力。最后,体外和体内结果表明 TAK-242 通过抑制 TLR4-p38 MAPK 信号发挥神经保护和抗铁死亡作用。在 HIBD 和 OGD 后,TLR4 激活诱导神经元铁死亡。抑制 TLR4 可减轻氧化应激引起的损伤,减少铁死亡的激活,并减轻 HIBD 后的神经炎症。在这项研究中,











































 京公网安备 11010802027423号
京公网安备 11010802027423号