Chemical & Pharmaceutical Bulletin ( IF 1.5 ) Pub Date : 2021-08-01 , DOI: 10.1248/cpb.c21-00148 Kazuaki Fukushima 1 , Hiroyoshi Esaki 1
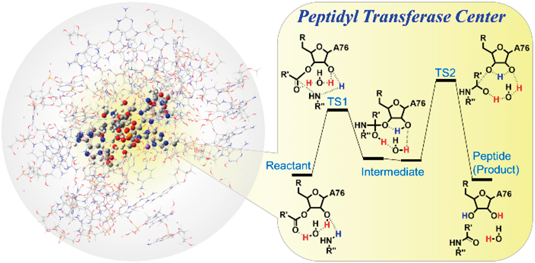
Peptide bond formation in living cells occurs at the peptidyl transferase center (PTC) of the large ribosomal subunit and involves the transfer of the peptidyl group from peptidyl-tRNA to aminoacyl-tRNA. Despite numerous kinetic and theoretical studies, many details of this reaction —such as whether it proceeds via a stepwise or concerted mechanism— remain unclear. In this study, we calculated the geometry and energy of the transition states and intermediates in peptide bond formation in the PTC environment using the ONIOM (our own n-layered integrated molecular orbital and molecular mechanics) method. The calculations indicated that the energy of the transition states of stepwise mechanisms are lower than those of concerted mechanisms and suggested that the reaction involves a neutral tetrahedral intermediate that is stabilized through the hydrogen-bonding network in the PTC environment. The results will lead to a better understanding of the mechanism of peptidyl transfer reaction, and resolve fundamental questions of the steps and molecular intermediates involved in peptide bond formation in the ribosome.
 Fullsize Image
Fullsize Image
中文翻译:

使用ONIOM方法进行核糖体肽键形成机制的理论研究
活细胞中肽键的形成发生在核糖体大亚基的肽基转移酶中心 (PTC),涉及肽基从肽基-tRNA 到氨酰基-tRNA 的转移。尽管进行了大量动力学和理论研究,但该反应的许多细节——例如它是通过逐步还是协同机制进行的——仍不清楚。在这项研究中,我们使用 ONIOM(我们自己的n-分层综合分子轨道和分子力学)方法。计算表明,逐步机制的过渡态能量低于协调机制的能量,并表明该反应涉及中性四面体中间体,该中间体通过 PTC 环境中的氢键网络稳定。该结果将有助于更好地理解肽基转移反应的机制,并解决核糖体中肽键形成所涉及的步骤和分子中间体的基本问题。
 全尺寸图像
全尺寸图像











































 京公网安备 11010802027423号
京公网安备 11010802027423号