Journal of Rare Earths ( IF 5.2 ) Pub Date : 2021-07-30 , DOI: 10.1016/j.jre.2021.07.013 Yumei Li 1, 2 , Jian Fan 2 , Xiaonan Feng 1 , Tao Tao 1
|
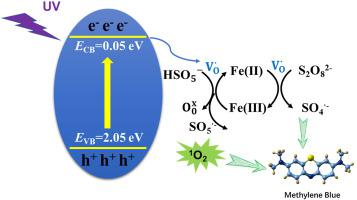
ABO3-type perovskite oxides with abounding defect structures and diverse physical chemistry attributes have been extensively studied in heterogeneous catalysis. Semiconductor LaFeO3 perovskites fabricated via the sol–gel method were used as peroxydisulfate (PDS) activators for methylene blue (MB) degradation under low-intensity ultra-violet (UV)-light to evaluate the degradation efficiency of organic pollutants and mechanism. The results indicate that under low-intensity UV irradiation, the developed UV/LaFeO3/PDS system shows excellent degradation ability of organic pollutants in a wide pH range. Electron spin resonance and radical quenching experiments verify that SO4·−, •OH, h+ and 1O2 are generated during the activation process, and 1O2 plays a dominant role in MB degradation. Reduction of low oxidation state Fe(II) and O22−/O− on the LaFeO3 surface shows that oxygen vacancy, as the electron transfer mediator, enhances the redox cycle efficiency of Fe(II)/Fe(III). Photogenerated electrons of LaFeO3 involved in the cyclic transformation of Fe(II)/Fe(III) and PDS activation further increase the number of active radicals and thus promote the synergistic effect of photocatalytic coupled with sulfate radical-based advanced oxidation processes.
中文翻译:

低强度紫外光照射下 LaFeO3 作为过硫酸盐活化剂降解有机物的催化性能和机理
ABO 3型钙钛矿氧化物具有丰富的缺陷结构和多种物理化学属性,已在多相催化中得到广泛研究。采用溶胶-凝胶法制备的半导体 LaFeO 3钙钛矿作为过二硫酸盐 (PDS) 活化剂在低强度紫外 (UV) 光下降解亚甲蓝 (MB) 以评估有机污染物的降解效率和机理。结果表明,在低强度紫外线照射下,所开发的UV/LaFeO 3 /PDS体系在较宽的pH范围内表现出优异的有机污染物降解能力。电子自旋共振和自由基猝灭实验验证了 SO 4 ·-、·OH、h +和1 O 2在活化过程中产生,1 O 2在MB降解中起主导作用。LaFeO 3表面上低氧化态Fe(II)和O 2 2- /O -的还原表明氧空位作为电子转移介质提高了Fe(II)/Fe(III)的氧化还原循环效率。参与 Fe(II)/Fe(III) 循环转化和 PDS 活化的 LaFeO 3的光生电子进一步增加了活性自由基的数量,从而促进了光催化与硫酸根基高级氧化过程的协同效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号