当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of diazonium chemistry in purine modifications: A focused review
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2021-07-31 , DOI: 10.1002/jhet.4352 Saumitra Sengupta 1 , Parthasarathi Das 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2021-07-31 , DOI: 10.1002/jhet.4352 Saumitra Sengupta 1 , Parthasarathi Das 1
Affiliation
|
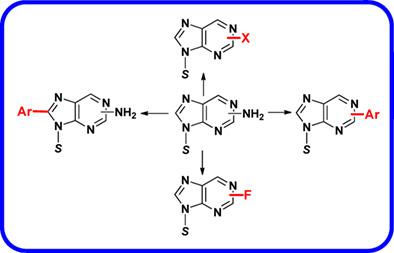
Deaminative modifications of purines via diazonium chemistry have long been the cornerstone in modified nucleoside synthesis. The purpose of this review is to bring into focus the impact of dediazoniative methods such as non-aqueous Sandmeyer halogenations, fluorination, arylation, and carbon-heteroatom bond formations on natural purines and their nucleosides as versatile tools for purine modifications en route to various nucleoside drugs, drug discovery scaffolds, and adducted oligonucleotides of biomedical interests. Metal free reactions of arenediazonium salts with purine nucleosides for the synthesis of 8-aryl purines, in particular, 8-aryl guanosines and photochromic 8-arylazo purines are also highlighted. The treatise is expected to create broad interest among organic and biological chemists for further explorations in this area.
中文翻译:

重氮化学在嘌呤修饰中的应用:重点综述
通过重氮化学对嘌呤进行脱氨基修饰长期以来一直是修饰核苷合成的基石。这篇综述的目的是关注去重氮方法的影响,例如非水性 Sandmeyer 卤化、氟化、芳基化和碳-杂原子键形成对天然嘌呤及其核苷的影响,这些方法作为嘌呤修饰的通用工具,可用于各种核苷的过程药物,药物发现支架和生物医学利益的加合物寡核苷酸。芳基重氮盐与嘌呤核苷的无金属反应用于合成 8-芳基嘌呤,特别是 8-芳基鸟苷和光致变色 8-芳基偶氮嘌呤。该论文有望在有机和生物化学家中引起广泛兴趣,以进一步探索该领域。
更新日期:2021-07-31
中文翻译:

重氮化学在嘌呤修饰中的应用:重点综述
通过重氮化学对嘌呤进行脱氨基修饰长期以来一直是修饰核苷合成的基石。这篇综述的目的是关注去重氮方法的影响,例如非水性 Sandmeyer 卤化、氟化、芳基化和碳-杂原子键形成对天然嘌呤及其核苷的影响,这些方法作为嘌呤修饰的通用工具,可用于各种核苷的过程药物,药物发现支架和生物医学利益的加合物寡核苷酸。芳基重氮盐与嘌呤核苷的无金属反应用于合成 8-芳基嘌呤,特别是 8-芳基鸟苷和光致变色 8-芳基偶氮嘌呤。该论文有望在有机和生物化学家中引起广泛兴趣,以进一步探索该领域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号