Computers in Biology and Medicine ( IF 7.0 ) Pub Date : 2021-07-31 , DOI: 10.1016/j.compbiomed.2021.104719 Claudia Guadalupe Benítez-Cardoza 1 , José Luis Vique-Sánchez 2
|
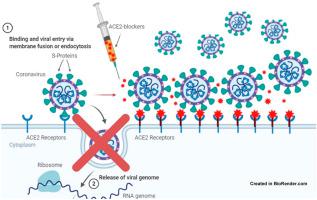
We investigated compounds selected by molecular docking to identify a specific treatment for COVID-19 that decreases the interaction between angiotensin-converting enzyme 2 (ACE2) and the receptor-binding domain (RBD) of SARS-CoV-2. Five compounds that interact with ACE2 amino acids Gln24, Asp30, His34, Tyr41, Gln42, Met82, Lys353, and Arg357 were evaluated using specific binding assays for their effects on the interaction between ACE2 with RBD. The compound labeled ED demonstrated favorable ACE2-binding, with an IC50 of 31.95 μM. ED cytotoxicity, evaluated using PC3 cells in an MTT assay, was consistent with the low theoretical toxicity previously reported. We propose that ED mainly interacts with His34, Glu37, and Lys353 in ACE2 and that it has an inhibitory effect on the interaction of ACE2 with the RBD of the S-protein. We recommend further investigation to develop ED into a potential drug or adjuvant in COVID-19 treatment.
中文翻译:

鉴定阻止 SARS-CoV-2 S 蛋白与 ACE2 结合的化合物
我们研究了通过分子对接选择的化合物,以确定针对 COVID-19 的特异性治疗方法,该治疗方法可减少血管紧张素转换酶 2 (ACE2) 与 SARS-CoV-2 受体结合域 (RBD) 之间的相互作用。使用特异性结合测定评估了与 ACE2 氨基酸 Gln24、Asp30、His34、Tyr41、Gln42、Met82、Lys353 和 Arg357 相互作用的五种化合物对 ACE2 与 RBD 之间相互作用的影响。标记为 ED 的化合物表现出良好的 ACE2 结合作用,IC 50为 31.95 μM。在 MTT 测定中使用 PC3 细胞评估的 ED 细胞毒性与先前报道的低理论毒性一致。我们认为ED主要与ACE2中的His34、Glu37和Lys353相互作用,并且对ACE2与S蛋白的RBD的相互作用具有抑制作用。我们建议进一步研究,将 ED 开发成潜在的药物或辅助治疗 COVID-19 的药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号