Journal of Advanced Research ( IF 11.4 ) Pub Date : 2021-07-30 , DOI: 10.1016/j.jare.2021.07.010 Min Mu 1 , Haifeng Chen 1 , Rangrang Fan 1 , Yuelong Wang 1 , Xin Tang 1 , Lan Mei 1 , Na Zhao 2 , Bingwen Zou 1 , Aiping Tong 1 , Jianguo Xu 1 , Bo Han 2 , Gang Guo 1
|
Introduction
Numerous options for treatment of glioblastoma have been explored; however, single-drug therapies and poor targeting have failed to provide effective drugs. Chemotherapy has significant antitumor effect, but the efficacy of single-drug therapies in the clinic is limited over a long period of time. Thus, novel therapeutic approaches are necessary to address these critical issues.
Objectives
The present study, we investigated a tumor-specific metal-tea polyphenol-based cascade nanoreactor for chemodynamic therapy-enhanced chemotherapy.
Methods
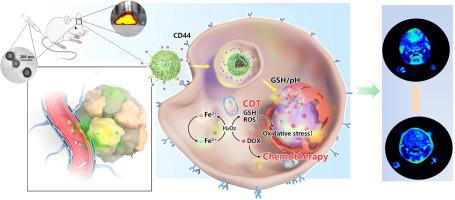
HA-EGCG was synthesized for the first time by introducing epigallocatechin-3-gallate (EGCG) into the skeleton of hyaluronic acid (HA) with reducible disulfide bonds. A rapid and green method was developed to fabricate the metal-tea polyphenol networks (MTP) with an HA-EGCG coating (DOX@MTP/HA-EGCG) based on Fe3+ and EGCG for targeted delivery of doxorubicin hydrochloride (DOX). GL261 cells were used to evaluate the antitumor efficacy of the DOX@MTP/HA-EGCG nanoreactor in vitro and in vivo.
Results
DOX@MTP/HA-EGCG nanoreactors were able to disassemble, resulting in escape of their components from lysosomes and precise release of DOX, Fe3+, and EGCG in the tumor cells. HA-EGCG depleted glutathione to amplify oxidative stress and enhance chemodynamic therapy. The results of in vivo experiments suggested that DOX@MTP/HA-EGCG specifically accumulates at the CD44-overexpressing GL261 tumor sites and that sustained release of DOX and Fe3+ induced a distinct therapeutic outcome.
Conclusions
The findings suggested the developed nanoreactor has promising potential as a future GL261 glioblastoma therapy.
中文翻译:

用于胶质母细胞瘤治疗的肿瘤特异性铁配位表没食子儿茶素-3-没食子酸酯级联纳米反应器
介绍
已经探索了多种治疗胶质母细胞瘤的方案;然而,单一药物疗法和靶向性差未能提供有效的药物。化疗具有显着的抗肿瘤作用,但临床上单药治疗长期疗效有限。因此,需要新的治疗方法来解决这些关键问题。
目标
在本研究中,我们研究了一种基于肿瘤特异性金属茶多酚的级联纳米反应器,用于化学动力学疗法增强化疗。
方法
首次通过将表没食子儿茶素-3-没食子酸酯(EGCG)引入具有可还原二硫键的透明质酸(HA)骨架中合成了HA-EGCG。开发了一种快速、绿色的方法来制造具有基于Fe 3+和EGCG的HA-EGCG涂层(DOX@MTP/HA-EGCG)的金属茶多酚网络(MTP),用于盐酸阿霉素(DOX)的靶向递送。利用GL261细胞评价DOX@MTP/HA-EGCG纳米反应器的体外和体内抗肿瘤功效。
结果
DOX@MTP/HA-EGCG 纳米反应器能够分解,导致其成分从溶酶体中逸出,并在肿瘤细胞中精确释放 DOX、Fe 3+和 EGCG。HA-EGCG 消耗谷胱甘肽,以放大氧化应激并增强化学动力学治疗。体内实验结果表明,DOX@MTP/HA-EGCG 在 CD44 过表达的 GL261 肿瘤位点特异性积累,并且 DOX 和 Fe 3+的持续释放诱导了独特的治疗结果。
结论
研究结果表明,所开发的纳米反应器作为未来的 GL261 胶质母细胞瘤治疗具有广阔的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号