Journal of Advanced Research ( IF 11.4 ) Pub Date : 2021-07-30 , DOI: 10.1016/j.jare.2021.07.011 Chongming Wu 1 , Ying Zhao 2 , Yingying Zhang 2 , Yanan Yang 1 , Wenquan Su 2 , Yuanyuan Yang 2 , Le Sun 1 , Fang Zhang 1 , Jiaqi Yu 1 , Yaoxian Wang 2 , Peng Guo 1 , Baoli Zhu 3 , Shengxian Wu 2
|
Introduction
Gut microbiota has been implicated in the pharmacological activities of many natural products. As an effective hypolipidemic agent, berberine (BBR)’s clinical application is greatly impeded by the obvious inter-individual response variation. To date, little evidence exists on the causality between gut microbes and its therapeutic effects, and the linkage of bacteria alterations to the inter-individual response variation.
Objectives
This study aims to confirm the causal role of the gut microbiota in BBR’s anti-hyperlipidemic effect and identify key bacteria that can predict its effectiveness.
Methods
The correlation between gut microbiota and BBR’s inter-individual response variation was studied in hyperlipidemic patients. The causal role of gut microbes in BBR’s anti-hyperlipidemic effects was subsequently assessed by altered administration routes, co-treatment with antibiotics, fecal microbiota transplantation, and metagenomic analysis.
Results
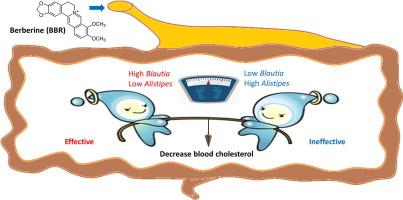
Three-month clinical study showed that BBR was effectively to decrease serum lipids but displayed an obvious response variation. The cholesterol-lowering but not triglyceride-decreasing effect of BBR was closely related to its modulation on gut microbiota. Interestingly, the baseline levels of Alistipes and Blautia could accurately predict its anti-hypercholesterolemic efficiency in the following treatment. Causality experiments in mice further confirmed that the gut microbiome is both necessary and sufficient to mediate the lipid-lowering effect of BBR. The absence of Blautia substantially abolished BBR's cholesterol-decreasing efficacy.
Conclusion
The gut microbiota is necessary and sufficient for BBR’s hyperlipidemia-ameliorating effect. The baseline composition of gut microbes can be an effective predictor for its pharmacotherapeutic efficacy, providing a novel way to achieve personalized therapy.
中文翻译:

肠道微生物群特异性介导小檗碱 (BBR) 的抗高胆固醇血症作用,并有助于预测 BBR 在患者中的降胆固醇功效
介绍
肠道微生物群与许多天然产物的药理活性有关。作为一种有效的降血脂药物,黄连素(BBR)的临床应用受到明显的个体间反应差异的极大阻碍。迄今为止,几乎没有证据表明肠道微生物与其治疗效果之间的因果关系,以及细菌改变与个体间反应变化之间的联系。
目标
本研究旨在确认肠道微生物群在 BBR 抗高血脂作用中的因果作用,并确定可以预测其有效性的关键细菌。
方法
在高脂血症患者中研究了肠道微生物群与 BBR 的个体间反应变异之间的相关性。随后通过改变给药途径、与抗生素联合治疗、粪便微生物群移植和宏基因组分析来评估肠道微生物在 BBR 抗高血脂作用中的因果作用。
结果
3个月的临床研究表明,BBR能有效降低血脂,但反应变化明显。BBR 降低胆固醇但不降低甘油三酯的作用与其对肠道微生物群的调节密切相关。有趣的是, Alistipes和Blautia的基线水平可以准确预测其在后续治疗中的抗高胆固醇血症效率。小鼠的因果关系实验进一步证实,肠道微生物组对于介导 BBR 的降脂作用既必要又足够。Blautia的缺失大大消除了 BBR 降低胆固醇的功效。
结论
肠道微生物群对于 BBR 的高脂血症改善作用是必要和充分的。肠道微生物的基线组成可以作为其药物治疗功效的有效预测指标,为实现个性化治疗提供了一种新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号