International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2021-07-31 , DOI: 10.1016/j.ijms.2021.116678 Douglas J.D. Pimlott 1 , Lars Konermann 1
|
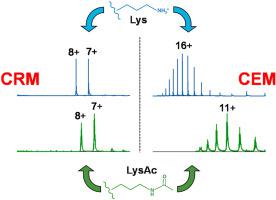
Different mechanisms have been proposed for the formation of gaseous protein ions during electrospray ionization (ESI). In the charged residue model (CRM) ions are produced upon nanodroplet evaporation to dryness. This mechanism is thought to dominate in native ESI, where proteins retain compact conformations, with charge states close to the Rayleigh charge of protein-sized aqueous droplets. Much higher charge states are generated from proteins that are unfolded in solution. The chain ejection model (CEM) has been proposed for ESI under such denaturing conditions. In the CEM proteins are gradually expelled, while mobile H+ equilibrate between the droplet and its protruding tail. Providing clear-cut evidence for these scenarios remains difficult, because electrosprayed ions do not usually retain any features that reveal their formation mechanism. In this work we propose that the stepwise elimination of basic sites can serve to distinguish between the CRM and CEM. Using cytochrome c as a model system, we studied proteins that had between zero and 19 Lys blocked by acetylation. In native ESI (pH 7) the same low charge states were observed regardless of acetylation. This behavior is consistent with the CRM, where charge states are governed by protein size, rather than protein surface chemistry. Denaturing (pH 2) conditions resulted in much higher ESI charge states. Intriguingly, spectra acquired under these pH 2 conditions gradually shifted to lower charge states when the number of acetylated Lys was increased. This charge reduction is attributed to the fact that lowering the number of basic sites compromises the ability of the protein to compete with the droplet for mobile H+ during the CEM. In conclusion, we illustrate that simple covalent modifications can help distinguish between protein ion formation via the CRM or the CEM.
中文翻译:

使用共价修饰区分蛋白质电喷雾机制:带电残留模型 (CRM) 与链喷射模型 (CEM)
已经提出了在电喷雾电离 (ESI) 过程中形成气态蛋白质离子的不同机制。在带电残留模型 (CRM) 中,纳米液滴蒸发至干后会产生离子。这种机制被认为在天然 ESI 中占主导地位,其中蛋白质保持紧凑的构象,电荷状态接近蛋白质大小的水滴的瑞利电荷。从在溶液中展开的蛋白质产生更高的电荷态。在这种变性条件下,已经为 ESI 提出了链喷射模型 (CEM)。在 CEM 中,蛋白质逐渐被排出,而移动的 H +在液滴与其突出的尾部之间保持平衡。为这些场景提供明确的证据仍然很困难,因为电喷雾离子通常不会保留任何揭示其形成机制的特征。在这项工作中,我们建议逐步消除基本站点可以用于区分 CRM 和 CEM。使用细胞色素c作为模型系统,我们研究了被乙酰化阻断的 0 到 19 个赖氨酸的蛋白质。在天然 ESI (pH 7) 中,无论乙酰化如何,都观察到相同的低电荷态。这种行为与 CRM 一致,其中电荷状态受蛋白质大小而不是蛋白质表面化学控制。变性 (pH 2) 条件导致更高的 ESI 电荷态。有趣的是,当乙酰化赖氨酸的数量增加时,在这些 pH 2 条件下获得的光谱逐渐转移到较低的电荷状态。这种电荷减少归因于这样一个事实,即降低碱性位点的数量会损害蛋白质与液滴竞争移动 H + 的能力在 CEM 期间。总之,我们说明简单的共价修饰可以帮助区分通过 CRM 或 CEM 形成的蛋白质离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号