Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2021-07-31 , DOI: 10.1016/j.bbadis.2021.166234 Josephine C Esposto 1 , Sanela Martic 2
|
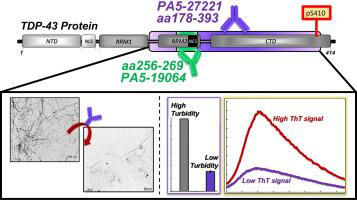
TAR DNA-binding protein-43 (TDP-43) pathology, including fibrillar aggregates and mutations, develops in amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD) and limbic-predominant age-related TDP-43 encephalopathy (LATE). Hyperphosphorylation and aggregation of TDP-43 contribute to pathology and are viable therapeutic targets for ALS. In vivo inhibition of TDP-43 aggregation was evaluated using anti-TDP-43 antibodies with promising outcomes. However, the exact mechanism of antibody-based inhibition targeting TDP-43 is not well understood but may lead to the identification of viable immunotherapies. Herein, the mechanism of in vitro aggregation of phosphorylated TDP-43 was explored, and the anti-TDP-43 antibodies tested for their inhibitor efficacies. Specifically, the aggregation of phosphorylated full-length TDP-43 protein (pS410) was monitored by transmission electron microscopy (TEM), turbidity absorbance, and thioflavin (ThT) spectroscopy. The protein aggregates were insoluble, ThT-positive and characterized with heterogeneous morphologies (fibers, amorphous structures). Antibodies specific to epitopes 178-393 and 256-269, within the RRM2-CTD domain, reduced the formation of β-sheets and insoluble aggregates, at low antibody loading (antibody: protein ratio = 1 μg/mL: 45 μg/mL). Inhibition outcomes were highly dependent on the type and loading of antibodies, indicating dual functionality of such inhibitors, as aggregation inhibitors or aggregation promoters. Anti-SOD1 and anti-tau antibodies were not effective inhibitors against TDP-43 aggregation, indicating selective inhibition.
中文翻译:

磷酸化焦油 DNA 结合蛋白 43:聚集和基于抗体的抑制
TAR DNA 结合蛋白 43 (TDP-43) 病理,包括纤维状聚集体和突变,在肌萎缩侧索硬化 (ALS)、额颞叶变性 (FTLD) 和边缘系统占优势的年龄相关性 TDP-43 脑病 (LATE) 中发展。TDP-43 的过度磷酸化和聚集有助于病理学,并且是 ALS 的可行治疗靶点。使用抗 TDP-43 抗体评估了 TDP-43 聚集的体内抑制,结果很有希望。然而,基于抗体的抑制靶向 TDP-43 的确切机制尚不清楚,但可能会导致确定可行的免疫疗法。在此,体外作用机制研究了磷酸化 TDP-43 的聚集,并测试了抗 TDP-43 抗体的抑制剂功效。具体而言,磷酸化全长 TDP-43 蛋白 (pS410) 的聚集通过透射电子显微镜 (TEM)、浊度吸光度和硫代黄素 (ThT) 光谱进行监测。蛋白质聚集体是不溶性的、ThT 阳性的并且具有异质形态(纤维、无定形结构)的特征。在 RRM2-CTD 结构域内,针对表位 178-393 和 256-269 的特异性抗体减少了 β-折叠和不溶性聚集体的形成,在低抗体负载下(抗体:蛋白质比率 = 1 μg/mL:45 μg/mL) . 抑制结果高度依赖于抗体的类型和负载,表明此类抑制剂具有双重功能,如聚集抑制剂或聚集促进剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号