Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2021-07-31 , DOI: 10.1016/j.bbagen.2021.129970 Mark A Rosenfeld 1 , Lyubov A Wasserman 1 , Alexandra D Vasilyeva 1 , Nadezhda A Podoplelova 2 , Mikhail A Panteleev 2 , Lyubov V Yurina 1
|
Background
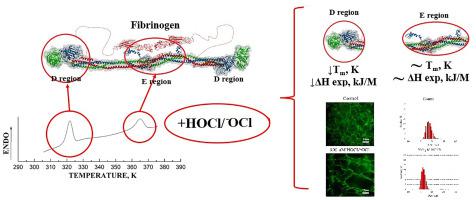
Human fibrinogen, which plays a key role in plasma haemostasis, is a highly vulnerable target for oxidants. Fibrinogen undergoes posttranslational modifications that can potentially disrupt protein structure and function.
Methods
For the first time, by differential scanning calorimetry, dynamic and elastic light scattering and confocal laser scanning microscopy, the consequences of HOCl/−OCl-induced oxidation of fibrinogen on its thermal denaturation, molecular size distribution and fibrin clot network have been explored.
Results
Within a wide range of HOCl/−OCl concentrations (50–300 μM), the molecular size distribution remained unimodal; however, the average size of the hydrated molecules decreased. HOCl/−OCl-induced oxidation of fibrinogen resulted in the diminished thermal stability of regions D and E. As evidenced by elastic light scattering and confocal laser scanning microscopy, HOCl/−OCl caused the formation of abnormal fibrin with a decreased diameter of individual fibres.
Conclusions
The current results along with data from previous studies enable one to conclude that the effect of HOCl/−OCl-mediated oxidation on the thermal stability of region D is influenced directly by oxidative damage to the D region structure. Since the E region is not subjected to oxidative modification, its structural damage is likely to be mediated by the oxidation of other protein structures, in particular α-helical coiled-coils.
General significance
The experimental findings acquired in the current study could help to elucidate the consequences of oxidative stress in vivo on damage to the structure of fibrinogen/fibrin under the action of different ROS species.
中文翻译:

次氯酸盐诱导的纤维蛋白原氧化:对其热变性和纤维蛋白结构的影响
背景
在血浆止血中起关键作用的人纤维蛋白原是氧化剂的一个非常脆弱的目标。纤维蛋白原经历可能破坏蛋白质结构和功能的翻译后修饰。
方法
首次,由差示扫描量热法,动态和弹性光散射和共聚焦激光扫描显微镜,次氯酸的后果/ - OCL诱导在它的热变性的纤维蛋白原的氧化,分子尺寸分布和纤维蛋白凝块网络已探索。
结果
在很宽的 HOCl/ - OCl 浓度范围内(50-300 μM),分子大小分布保持单峰;然而,水合分子的平均尺寸减小了。HOCl/ - OCl 诱导的纤维蛋白原氧化导致区域 D 和 E 的热稳定性降低。 正如弹性光散射和共聚焦激光扫描显微镜所证明的那样,HOCl/ - OCl 导致异常纤维蛋白的形成,单个纤维的直径减小.
结论
当前的结果以及先前研究的数据使人们能够得出结论,HOCl / - OCl 介导的氧化对 D 区热稳定性的影响直接受到 D 区结构氧化损伤的影响。由于 E 区不受氧化修饰,其结构损伤可能是由其他蛋白质结构的氧化介导的,特别是 α-螺旋卷曲螺旋。
一般意义
当前研究中获得的实验结果有助于阐明体内氧化应激对不同活性氧作用下纤维蛋白原/纤维蛋白结构损伤的后果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号