Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Novel Stimuli-Responsive Injectable Antibacterial Hydrogel to Achieve Synergetic Photothermal/Gene-Targeted Therapy towards Uveal Melanoma
Advanced Science ( IF 14.3 ) Pub Date : 2021-07-31 , DOI: 10.1002/advs.202004721 Shaoyun Wang 1, 2 , Baohui Chen 3, 4 , Liping Ouyang 3 , Donghui Wang 3 , Ji Tan 3 , Yuqin Qiao 3, 5 , Shengfang Ge 1, 2 , Jing Ruan 1, 2 , Ai Zhuang 1, 2 , Xuanyong Liu 3, 4, 5, 6 , Renbing Jia 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2021-07-31 , DOI: 10.1002/advs.202004721 Shaoyun Wang 1, 2 , Baohui Chen 3, 4 , Liping Ouyang 3 , Donghui Wang 3 , Ji Tan 3 , Yuqin Qiao 3, 5 , Shengfang Ge 1, 2 , Jing Ruan 1, 2 , Ai Zhuang 1, 2 , Xuanyong Liu 3, 4, 5, 6 , Renbing Jia 1, 2
Affiliation
|
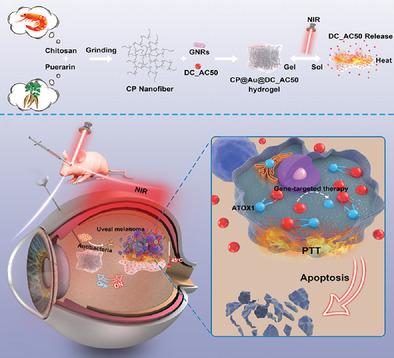
Uveal melanoma (UM) is the most prevalent primary intraocular malignant tumor with a high lethal rate. Patients who undergo conventional enucleation treatments consistently suffer permanent blindness, facial defects, and mental disorders, therefore, novel therapeutic modalities are urgently required. Herein, an injectable and stimuli-responsive drug delivery antibacterial hydrogel (CP@Au@DC_AC50) is constructed via a facile grinding method that is inspired by the preparation process of traditional Chinese medicine. The incorporation of gold nanorods can enhance the mechanical strength of the hydrogel and realize photothermal therapy (PTT) and thermosensitive gel-sol transformation to release the gene-targeted drug DC_AC50 on demand in response to low-density near-infrared (NIR) light. The orthotopic model of UM is built successfully and indicates the excellent efficiency of CP@Au@DC_AC50 in killing tumors without damage to normal tissue because of its synergistic mild temperature PTT and gene-targeted therapy. Moreover, the eyeball infection model reveals the remarkable antibacterial properties of the hydrogel which can prevent endophthalmitis in the eyeball. There is negligible difference between the CP@Au@DC_AC50+NIR group and normal group. This NIR light-triggered gene-targeted therapy/PTT/antibacterial treatment pattern provides a promising strategy for building multifunctional therapeutic platform against intraocular tumors and exhibits great potential for the clinical treatment of UM.
中文翻译:

一种新型刺激响应型可注射抗菌水凝胶,可实现葡萄膜黑色素瘤的光热/基因靶向协同治疗
葡萄膜黑色素瘤(UM)是最常见的原发性眼内恶性肿瘤,致死率很高。接受传统剜除术治疗的患者始终患有永久性失明、面部缺陷和精神障碍,因此迫切需要新的治疗方式。在此,受中药制备过程启发,通过简单的研磨方法构建了一种可注射和刺激响应的药物递送抗菌水凝胶(CP@Au@DC_AC50)。金纳米棒的掺入可以增强水凝胶的机械强度,并实现光热疗法(PTT)和热敏凝胶溶胶转化,响应低密度近红外(NIR)光按需释放基因靶向药物DC_AC50。成功建立UM原位模型,表明CP@Au@DC_AC50在不损伤正常组织的情况下具有优异的杀伤肿瘤效率,因为其协同温和的PTT和基因靶向治疗。此外,眼球感染模型揭示了水凝胶显着的抗菌特性,可以预防眼球内眼内炎。CP@Au@DC_AC50+NIR组和正常组之间的差异可以忽略不计。这种近红外光触发基因靶向治疗/PTT/抗菌治疗模式为构建针对眼内肿瘤的多功能治疗平台提供了一种有前景的策略,并在UM的临床治疗中表现出巨大的潜力。
更新日期:2021-09-22
中文翻译:

一种新型刺激响应型可注射抗菌水凝胶,可实现葡萄膜黑色素瘤的光热/基因靶向协同治疗
葡萄膜黑色素瘤(UM)是最常见的原发性眼内恶性肿瘤,致死率很高。接受传统剜除术治疗的患者始终患有永久性失明、面部缺陷和精神障碍,因此迫切需要新的治疗方式。在此,受中药制备过程启发,通过简单的研磨方法构建了一种可注射和刺激响应的药物递送抗菌水凝胶(CP@Au@DC_AC50)。金纳米棒的掺入可以增强水凝胶的机械强度,并实现光热疗法(PTT)和热敏凝胶溶胶转化,响应低密度近红外(NIR)光按需释放基因靶向药物DC_AC50。成功建立UM原位模型,表明CP@Au@DC_AC50在不损伤正常组织的情况下具有优异的杀伤肿瘤效率,因为其协同温和的PTT和基因靶向治疗。此外,眼球感染模型揭示了水凝胶显着的抗菌特性,可以预防眼球内眼内炎。CP@Au@DC_AC50+NIR组和正常组之间的差异可以忽略不计。这种近红外光触发基因靶向治疗/PTT/抗菌治疗模式为构建针对眼内肿瘤的多功能治疗平台提供了一种有前景的策略,并在UM的临床治疗中表现出巨大的潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号