当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the ligand binding specificity of SREC-II (scavenger receptor expressed by endothelial cells)
FEBS Open Bio ( IF 2.6 ) Pub Date : 2021-07-30 , DOI: 10.1002/2211-5463.13260 Catherine Wicker-Planquart 1 , Pascale Tacnet-Delorme 1 , Laurence Preisser 2 , Samy Dufour 1 , Yves Delneste 2 , Dominique Housset 1 , Philippe Frachet 1 , Nicole M Thielens 1
FEBS Open Bio ( IF 2.6 ) Pub Date : 2021-07-30 , DOI: 10.1002/2211-5463.13260 Catherine Wicker-Planquart 1 , Pascale Tacnet-Delorme 1 , Laurence Preisser 2 , Samy Dufour 1 , Yves Delneste 2 , Dominique Housset 1 , Philippe Frachet 1 , Nicole M Thielens 1
Affiliation
|
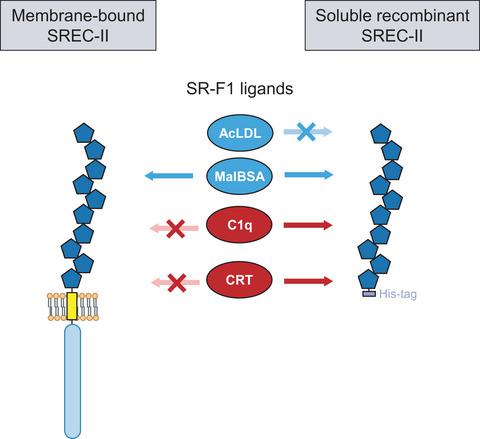
SREC-II (scavenger receptor expressed by endothelial cells II) is a membrane protein encoded by the SCARF2 gene, with high homology to class F scavenger receptor SR-F1, but no known scavenging function. We produced the extracellular domain of SREC-II in a recombinant form and investigated its capacity to interact with common scavenger receptor ligands, including acetylated low-density lipoprotein (AcLDL) and maleylated or acetylated BSA (MalBSA or AcBSA). Whereas no binding was observed for AcLDL, SREC-II ectodomain interacted strongly with MalBSA and bound with high affinity to AcBSA, a property shared with the SR-F1 ectodomain. SREC-II ectodomain also interacted with two SR-F1-specific ligands, complement C1q and calreticulin, with affinities in the 100 nm range. We proceeded to generate a stable CHO cell line overexpressing full-length SREC-II; binding of MalBSA to these cells was significantly increased compared with nontransfected CHO cells. In contrast, no increase in binding could be detected for C1q and calreticulin. We show for the first time that SREC-II has the capacity to interact with the common scavenger receptor ligand MalBSA. In addition, our data highlight similarities and differences in the ligand binding properties of SREC-II in soluble form and at the cell surface, and show that endogenous protein ligands of the ectodomain of SREC-II, such as C1q and calreticulin, are shared with the corresponding domain of SR-F1.
中文翻译:

深入了解 SREC-II(内皮细胞表达的清道夫受体)的配体结合特异性
SREC-II(由内皮细胞II表达的清道夫受体)是一种由SCARF2基因编码的膜蛋白,与F类清道夫受体SR-F1具有高度同源性,但没有已知的清除功能。我们以重组形式生产了 SREC-II 的细胞外结构域,并研究了其与常见清道夫受体配体相互作用的能力,包括乙酰化低密度脂蛋白 (AcLDL) 和马来酰化或乙酰化 BSA (MalBSA 或 AcBSA)。虽然没有观察到 AcLDL 的结合,但 SREC-II 胞外域与 MalBSA 强烈相互作用,并与 AcBSA 具有高亲和力,这是与 SR-F1 胞外域共有的特性。SREC-II 胞外域还与两个 SR-F1 特异性配体相互作用,补体 C1q 和钙网蛋白,亲和力为 100 nm范围。我们继续生成过表达全长 SREC-II 的稳定 CHO 细胞系;与未转染的 CHO 细胞相比,MalBSA 与这些细胞的结合显着增加。相反,没有检测到 C1q 和钙网蛋白的结合增加。我们首次表明 SREC-II 具有与常见的清道夫受体配体 MalBSA 相互作用的能力。此外,我们的数据突出了 SREC-II 在可溶形式和细胞表面的配体结合特性的相似性和差异,并表明 SREC-II 胞外域的内源性蛋白配体,如 C1q 和钙网蛋白,与SR-F1 的对应域。
更新日期:2021-10-02
中文翻译:

深入了解 SREC-II(内皮细胞表达的清道夫受体)的配体结合特异性
SREC-II(由内皮细胞II表达的清道夫受体)是一种由SCARF2基因编码的膜蛋白,与F类清道夫受体SR-F1具有高度同源性,但没有已知的清除功能。我们以重组形式生产了 SREC-II 的细胞外结构域,并研究了其与常见清道夫受体配体相互作用的能力,包括乙酰化低密度脂蛋白 (AcLDL) 和马来酰化或乙酰化 BSA (MalBSA 或 AcBSA)。虽然没有观察到 AcLDL 的结合,但 SREC-II 胞外域与 MalBSA 强烈相互作用,并与 AcBSA 具有高亲和力,这是与 SR-F1 胞外域共有的特性。SREC-II 胞外域还与两个 SR-F1 特异性配体相互作用,补体 C1q 和钙网蛋白,亲和力为 100 nm范围。我们继续生成过表达全长 SREC-II 的稳定 CHO 细胞系;与未转染的 CHO 细胞相比,MalBSA 与这些细胞的结合显着增加。相反,没有检测到 C1q 和钙网蛋白的结合增加。我们首次表明 SREC-II 具有与常见的清道夫受体配体 MalBSA 相互作用的能力。此外,我们的数据突出了 SREC-II 在可溶形式和细胞表面的配体结合特性的相似性和差异,并表明 SREC-II 胞外域的内源性蛋白配体,如 C1q 和钙网蛋白,与SR-F1 的对应域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号