Process Biochemistry ( IF 3.7 ) Pub Date : 2021-07-30 , DOI: 10.1016/j.procbio.2021.07.022 Larissa Alves Lopes 1 , Lucas Pinheiro Dias 2 , Helen Paula Silva da Costa 1 , João Xavier da Silva Neto 1 , Eva Gomes Morais 1 , José Tadeu Abreu de Oliveira 1 , Ilka Maria Vasconcelos 1 , Daniele de Oliveira Bezerra de Sousa 1
|
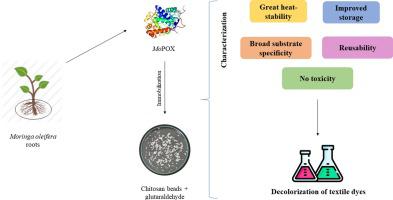
The textile industry is essential, but it is also responsible for causing environmental problems, particularly the discharge of dyes. In this context, this study aimed to immobilize a previously purified peroxidase, called MoPOX, on glutaraldehyde-activated chitosan beads in order to improve the potential for textile dye decolorization. The chitosan beads were activated with 8% glutaraldehyde for 1 h, and the immobilization was performed at 30 °C, pH 5.2 for 4 h. Scanning Electron Microscopy (SEM) analyses were used to observe the differences in the chitosan beads after immobilization. The optimum temperature dropped from 70 °C to 30 °C after immobilization, but immobilized MoPOX demonstrated excellent heat stability. The optimum pH remained 5.2, while the apparent kinetic constant value (Km) of immobilized MoPOX (14.67 mM) was lower in comparison to free MoPOX (46.8 mM). The immobilized enzyme showed improved activity after long storage times, and it could retain 40 % of its original activity even after 5 cycles. The potential for decolorization of different textile dyes was considerably enhanced after immobilization, reaching more than 80 %. Also, MoPOX showed no toxicity towards Artemia salina. Overall, the findings point to the promising potential of using immobilized MoPOX as a biocatalyst in a variety of biotechnological applications.
中文翻译:

来自辣木的过氧化物酶的固定化。壳聚糖珠上的根 (MoPOX) 增强了纺织染料的脱色
纺织业是必不可少的,但它也造成了环境问题,尤其是染料的排放。在这种情况下,本研究旨在将一种先前纯化的过氧化物酶(称为Mo POX)固定在戊二醛活化的壳聚糖珠上,以提高纺织染料脱色的潜力。壳聚糖珠用 8% 戊二醛活化 1 小时,固定在 30 °C,pH 5.2 下进行 4 小时。使用扫描电子显微镜 (SEM) 分析来观察固定化后壳聚糖珠粒的差异。固定后最适温度从 70 °C 下降到 30 °C,但固定MoPOX 表现出优异的热稳定性。最佳 pH 值保持在 5.2,而固定化Mo POX (14.67 mM)的表观动力学常数值 (K m )低于游离Mo POX (46.8 mM)。固定化酶在长时间储存后显示出更高的活性,即使在 5 个循环后仍可保持其原始活性的 40%。不同纺织染料的脱色潜力在固定后显着增强,达到 80% 以上。此外,Mo POX 对卤虫没有表现出毒性。总体而言,这些发现表明在各种生物技术应用中使用固定化 MoPOX 作为生物催化剂具有广阔的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号