当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
L-DOPA Dioxygenase Activity on 6-Substituted Dopamine Analogues
Biochemistry ( IF 2.9 ) Pub Date : 2021-07-29 , DOI: 10.1021/acs.biochem.1c00310 Alexander M Goldberg 1 , Miranda K Robinson 1 , Erykah S Starr 2 , Ryan N Marasco 2 , Alexa C Alana 2 , C Skyler Cochrane 2 , Kameron L Klugh 2 , David J Strzeminski 1 , Muxue Du 1 , Keri L Colabroy 1 , Larryn W Peterson 2
Biochemistry ( IF 2.9 ) Pub Date : 2021-07-29 , DOI: 10.1021/acs.biochem.1c00310 Alexander M Goldberg 1 , Miranda K Robinson 1 , Erykah S Starr 2 , Ryan N Marasco 2 , Alexa C Alana 2 , C Skyler Cochrane 2 , Kameron L Klugh 2 , David J Strzeminski 1 , Muxue Du 1 , Keri L Colabroy 1 , Larryn W Peterson 2
Affiliation
|
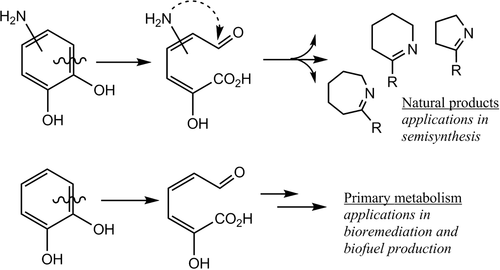
Dioxygenase enzymes are essential protein catalysts for the breakdown of catecholic rings, structural components of plant woody tissue. This powerful chemistry is used in nature to make antibiotics and other bioactive materials or degrade plant material, but we have a limited understanding of the breadth and depth of substrate space for these potent catalysts. Here we report steady-state and pre-steady-state kinetic analysis of dopamine derivatives substituted at the 6-position as substrates of L-DOPA dioxygenase, and an analysis of that activity as a function of the electron-withdrawing nature of the substituent. Steady-state and pre-steady-state kinetic data demonstrate the dopamines are impaired in binding and catalysis with respect to the cosubstrate molecular oxygen, which likely afforded spectroscopic observation of an early reaction intermediate, the semiquinone of dopamine. The reaction pathway of dopamine in the pre-steady state is consistent with a nonproductive mode of binding of oxygen at the active site. Despite these limitations, L-DOPA dioxygenase is capable of binding all of the dopamine derivatives and catalyzing multiple turnovers of ring cleavage for dopamine, 6-bromodopamine, 6-carboxydopamine, and 6-cyanodopamine. 6-Nitrodopamine was a single-turnover substrate. The variety of substrates accepted by the enzyme is consistent with an interplay of factors, including the capacity of the active site to bind large, negatively charged groups at the 6-position and the overall oxidizability of each catecholamine, and is indicative of the utility of extradiol cleavage in semisynthetic and bioremediation applications.
中文翻译:

L-DOPA 双加氧酶对 6-取代多巴胺类似物的活性
双加氧酶是分解植物木质组织的结构成分儿茶酚环的重要蛋白质催化剂。这种强大的化学物质在自然界中被用来制造抗生素和其他生物活性材料或降解植物材料,但我们对这些有效催化剂的底物空间的广度和深度了解有限。在这里,我们报告了在 6 位取代的多巴胺衍生物作为 L-多巴双加氧酶的底物的稳态和稳态动力学分析,以及作为取代基吸电子性质函数的该活性的分析。稳态和稳态前的动力学数据表明,多巴胺在结合和催化共底物分子氧方面受到损害,这可能提供了对早期反应中间体多巴胺半醌的光谱观察。多巴胺在稳态前的反应途径与活性位点氧结合的非生产模式一致。尽管有这些限制,L-DOPA 双加氧酶能够结合所有多巴胺衍生物,并催化多巴胺、6-溴多巴胺、6-羧基多巴胺和 6-氰多巴胺的多次环裂解转换。6-硝基多巴胺是单周转底物。酶接受的底物种类与多种因素的相互作用一致,包括活性位点在 6 位结合大的带负电荷基团的能力以及每种儿茶酚胺的整体氧化性,
更新日期:2021-08-17
中文翻译:

L-DOPA 双加氧酶对 6-取代多巴胺类似物的活性
双加氧酶是分解植物木质组织的结构成分儿茶酚环的重要蛋白质催化剂。这种强大的化学物质在自然界中被用来制造抗生素和其他生物活性材料或降解植物材料,但我们对这些有效催化剂的底物空间的广度和深度了解有限。在这里,我们报告了在 6 位取代的多巴胺衍生物作为 L-多巴双加氧酶的底物的稳态和稳态动力学分析,以及作为取代基吸电子性质函数的该活性的分析。稳态和稳态前的动力学数据表明,多巴胺在结合和催化共底物分子氧方面受到损害,这可能提供了对早期反应中间体多巴胺半醌的光谱观察。多巴胺在稳态前的反应途径与活性位点氧结合的非生产模式一致。尽管有这些限制,L-DOPA 双加氧酶能够结合所有多巴胺衍生物,并催化多巴胺、6-溴多巴胺、6-羧基多巴胺和 6-氰多巴胺的多次环裂解转换。6-硝基多巴胺是单周转底物。酶接受的底物种类与多种因素的相互作用一致,包括活性位点在 6 位结合大的带负电荷基团的能力以及每种儿茶酚胺的整体氧化性,









































 京公网安备 11010802027423号
京公网安备 11010802027423号