当前位置:
X-MOL 学术
›
ACS Chem. Health Saf.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lessons Learned─Lithium Silicide Hydration Fire
ACS Chemical Health & Safety Pub Date : 2021-07-28 , DOI: 10.1021/acs.chas.1c00040 Brynal A. Benally 1 , Benjamin W. Juba 1 , David Schafer 1 , Adam S. Pimentel 2 , Jessica K. Román-Kustas 1
ACS Chemical Health & Safety Pub Date : 2021-07-28 , DOI: 10.1021/acs.chas.1c00040 Brynal A. Benally 1 , Benjamin W. Juba 1 , David Schafer 1 , Adam S. Pimentel 2 , Jessica K. Román-Kustas 1
Affiliation
|
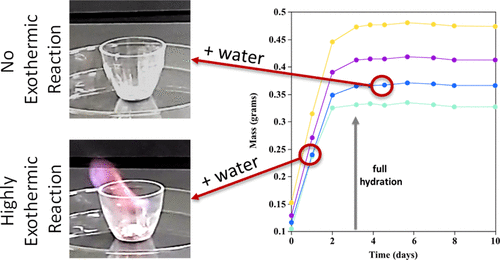
Alkali metals, such as lithium, sodium, potassium, etc., are highly reactive elements. While researchers generally handle these metals with caution, less caution is taken when these elements have been “reacted”. Here, a recent incident is examined in which a pair of researchers ignited a lithium silicide alloy sample that was assumed to be fully hydrated to lithium hydroxide and, thereby, no longer water-reactive. However, variations in the original chemical composition of the lithium compounds examined resulted in select mixtures failing to hydrate and react completely to lithium hydroxide in the time frame allowed. This gave rise to residual unreacted, water-sensitive lithium silicide which resulted in a violent exothermic reaction with water and autoignition of the produced hydrogen gas. This Article describes this incident and improvements that can be implemented to prevent similar incidents from occurring.
中文翻译:

经验教训─硅化锂水合火灾
碱金属,如锂、钠、钾等,是高活性元素。虽然研究人员通常会谨慎处理这些金属,但当这些元素“发生反应”时就不那么谨慎了。在这里,检查了最近发生的一起事件,其中一对研究人员点燃了硅化锂合金样品,该样品被认为已完全水合为氢氧化锂,因此不再与水反应。然而,所检查的锂化合物的原始化学成分的变化导致选择的混合物未能在允许的时间范围内水合并与氢氧化锂完全反应。这产生了残留的未反应的、对水敏感的硅化锂,导致与水发生剧烈放热反应和产生的氢气自燃。
更新日期:2021-07-28
中文翻译:

经验教训─硅化锂水合火灾
碱金属,如锂、钠、钾等,是高活性元素。虽然研究人员通常会谨慎处理这些金属,但当这些元素“发生反应”时就不那么谨慎了。在这里,检查了最近发生的一起事件,其中一对研究人员点燃了硅化锂合金样品,该样品被认为已完全水合为氢氧化锂,因此不再与水反应。然而,所检查的锂化合物的原始化学成分的变化导致选择的混合物未能在允许的时间范围内水合并与氢氧化锂完全反应。这产生了残留的未反应的、对水敏感的硅化锂,导致与水发生剧烈放热反应和产生的氢气自燃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号