当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gene-editing by CRISPR–Cas9 in combination with anthracycline therapy via tumor microenvironment-switchable, EGFR-targeted, and nucleus-directed nanoparticles for head and neck cancer suppression
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2021-07-29 , DOI: 10.1039/d1nh00254f Chen-Shen Wang, Chih-Hsien Chang, Tsai-Yu Tzeng, Anya Maan-Yuh Lin, Yu-Li Lo
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2021-07-29 , DOI: 10.1039/d1nh00254f Chen-Shen Wang, Chih-Hsien Chang, Tsai-Yu Tzeng, Anya Maan-Yuh Lin, Yu-Li Lo
|
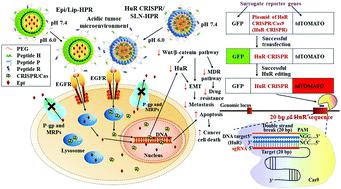
Head and neck cancer (HNC) has a high incidence and a poor prognosis. Epirubicin, a topoisomerase inhibitor, is a potential anthracycline chemotherapeutic for HNC treatment. HuR (ELAVL1), an RNA-binding protein, plays a critical role in promoting tumor survival, invasion, and resistance. HuR knockout via CRISPR/Cas9 (HuR CRISPR) is a possible strategy for the simultaneous modulation of the various pathways of tumor progression. Multifunctional nanoparticles modified with pH-sensitive epidermal growth factor receptor (EGFR)-targeting and nucleus-directed peptides were designed for the efficient delivery of HuR CRISPR and epirubicin to human tongue squamous carcinoma SAS cells and SAS tumor-bearing mice. The pH-sensitive nanoparticles responded to the acidic pH value as a switch to expose the targeting peptides. The cellular uptake and transfection efficiency of these nanoparticles in SAS cells increased via EGFR targeting, ligand-mediated endocytosis, and endosomal escape. These nanoparticles showed low cytotoxicity towards normal oral keratinocyte NOK cells. CRISPR/Cas9 was transported into the nucleus via the nuclear directing peptide and successfully knocked out HuR to suppress proliferation, metastasis, and resistance in SAS cells. The multiple inhibition of EGFR/β-catenin/epithelial–mesenchymal transition pathways was mediated through modulating the EGFR/PI3K/mTOR/AKT axis. The co-treatment of epirubicin and HuR CRISPR in SAS cells further facilitated apoptosis/necroptosis/autophagy and caused cancer cell death. In combination with HuR CRISPR nanoparticles, the efficacy and safety of epirubicin nanoparticles against cancer in SAS tumor-bearing mice improved significantly. Collectively, these nanoparticles showed a tumor pH response, active EGFR targeting, and nuclear localization and thus offered a combinatorial spatiotemporal platform for chemotherapy and the CRISPR/Cas gene-editing system.
中文翻译:

CRISPR-Cas9基因编辑结合蒽环类药物治疗,通过肿瘤微环境可切换、EGFR靶向和细胞核导向的纳米粒子抑制头颈癌
头颈癌(HNC)发病率高,预后差。表柔比星是一种拓扑异构酶抑制剂,是治疗 HNC 的潜在蒽环类化疗药物。HuR (ELAVL1) 是一种 RNA 结合蛋白,在促进肿瘤存活、侵袭和抵抗方面起着关键作用。HuR 淘汰赛通过CRISPR/Cas9 (HuR CRISPR) 是一种可能的策略,用于同时调节肿瘤进展的各种途径。用 pH 敏感表皮生长因子受体 (EGFR) 靶向和细胞核定向肽修饰的多功能纳米颗粒设计用于将 HuR CRISPR 和表柔比星有效递送至人舌鳞癌 SAS 细胞和 SAS 荷瘤小鼠。pH 敏感纳米颗粒响应酸性 pH 值作为暴露靶向肽的开关。这些纳米颗粒在 SAS 细胞中的细胞摄取和转染效率通过EGFR 靶向、配体介导的内吞作用和内体逃逸而增加。这些纳米颗粒对正常口腔角质形成细胞 NOK 细胞显示出低细胞毒性。CRISPR/Cas9被转运入细胞核通过核定向肽并成功敲除 HuR 以抑制 SAS 细胞的增殖、转移和抵抗。EGFR/β-catenin/上皮-间质转化通路的多重抑制是通过调节 EGFR/PI3K/mTOR/AKT 轴介导的。表柔比星和 HuR CRISPR 在 SAS 细胞中的共同处理进一步促进了细胞凋亡/坏死性凋亡/自噬并导致癌细胞死亡。与 HuR CRISPR 纳米颗粒相结合,表柔比星纳米颗粒在 SAS 荷瘤小鼠中抗癌的疗效和安全性显着提高。总的来说,这些纳米颗粒显示出肿瘤 pH 响应、主动 EGFR 靶向和核定位,从而为化疗和 CRISPR/Cas 基因编辑系统提供了一个组合时空平台。
更新日期:2021-07-29
中文翻译:

CRISPR-Cas9基因编辑结合蒽环类药物治疗,通过肿瘤微环境可切换、EGFR靶向和细胞核导向的纳米粒子抑制头颈癌
头颈癌(HNC)发病率高,预后差。表柔比星是一种拓扑异构酶抑制剂,是治疗 HNC 的潜在蒽环类化疗药物。HuR (ELAVL1) 是一种 RNA 结合蛋白,在促进肿瘤存活、侵袭和抵抗方面起着关键作用。HuR 淘汰赛通过CRISPR/Cas9 (HuR CRISPR) 是一种可能的策略,用于同时调节肿瘤进展的各种途径。用 pH 敏感表皮生长因子受体 (EGFR) 靶向和细胞核定向肽修饰的多功能纳米颗粒设计用于将 HuR CRISPR 和表柔比星有效递送至人舌鳞癌 SAS 细胞和 SAS 荷瘤小鼠。pH 敏感纳米颗粒响应酸性 pH 值作为暴露靶向肽的开关。这些纳米颗粒在 SAS 细胞中的细胞摄取和转染效率通过EGFR 靶向、配体介导的内吞作用和内体逃逸而增加。这些纳米颗粒对正常口腔角质形成细胞 NOK 细胞显示出低细胞毒性。CRISPR/Cas9被转运入细胞核通过核定向肽并成功敲除 HuR 以抑制 SAS 细胞的增殖、转移和抵抗。EGFR/β-catenin/上皮-间质转化通路的多重抑制是通过调节 EGFR/PI3K/mTOR/AKT 轴介导的。表柔比星和 HuR CRISPR 在 SAS 细胞中的共同处理进一步促进了细胞凋亡/坏死性凋亡/自噬并导致癌细胞死亡。与 HuR CRISPR 纳米颗粒相结合,表柔比星纳米颗粒在 SAS 荷瘤小鼠中抗癌的疗效和安全性显着提高。总的来说,这些纳米颗粒显示出肿瘤 pH 响应、主动 EGFR 靶向和核定位,从而为化疗和 CRISPR/Cas 基因编辑系统提供了一个组合时空平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号