Desalination ( IF 8.3 ) Pub Date : 2021-07-29 , DOI: 10.1016/j.desal.2021.115218 Muenir M. Besli 1, 2 , Saravanan Kuppan 1 , Sharon E. Bone 3 , Sami Sainio 3, 4 , Sondra Hellstrom 1 , Jake Christensen 1 , Michael Metzger 5
|
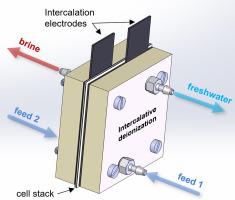
Intercalative deionization (IDI) uses two cation intercalation electrodes separated by an anion exchange membrane in a symmetric cell design that has the potential to deliver electrochemically desalinated water in an energy- and water-efficient way. Here, we define and measure metrics to describe the performance and lifetime of IDI cells and compare them for NaCl and CaCl2 feed solutions. With 20 mM NaCl, NiHCF/AEM/NiHCF flow cells achieve 10 mM average concentration change at a productivity of 20 l/h/m2 and 5 mM average concentration change at 130 l/h/m2. In both cases the cells are operated at a 3C current rate and consume ~30 Wh/m3 of energy. With 10 mM CaCl2, the specific capacity and salt removal of IDI flow cells is ~4 times lower. NiHCF/NiHCF beaker cells with CaCl2 electrolyte suffer from strong capacity fade, while the same cells with NaCl electrolyte achieve 500 cycles without any capacity fade. Our post-mortem analysis using X-ray diffraction, secondary electron microscopy, energy dispersive X-ray spectroscopy, synchrotron-based X-ray absorption spectroscopy and micro X-ray fluorescence mapping reveals that NiHCF dissolves upon repeated intercalation with Ca2+, releasing residual K+, Ni2+ and Fe(CN)63, which precipitates as a crystalline decomposition product on the electrodes. This side reaction deprives the active material NiHCF of charge compensating Fe, and thus accounts for the observed capacity fade.
中文翻译:

用于去除单价和二价离子的插入式去离子水池的性能和寿命
插层去离子 (IDI) 在对称池设计中使用由阴离子交换膜隔开的两个阳离子插层电极,该电极有可能以节能和节水的方式提供电化学脱盐水。在这里,我们定义和测量指标来描述 IDI 电池的性能和寿命,并将它们与 NaCl 和 CaCl 2进料溶液进行比较。使用 20 mM NaCl,NiHCF/AEM/NiHCF 流通池在 20 l/h/m 2的生产率下实现 10 mM 平均浓度变化,在 130 l/h/m 2 下实现5 mM 平均浓度变化。在这两种情况下,电池都以 3C 的电流速率运行并消耗约 30 Wh/m 3的能量。含 10 mM CaCl 2,IDI 流通池的比容量和除盐率低约 4 倍。使用 CaCl 2电解质的NiHCF/NiHCF 烧杯电池容量衰减严重,而使用 NaCl 电解质的相同电池可实现 500 次循环而没有任何容量衰减。我们使用 X 射线衍射、二次电子显微镜、能量色散 X 射线光谱、基于同步加速器的 X 射线吸收光谱和显微 X 射线荧光图的验尸分析表明,NiHCF 在与 Ca 2+重复嵌入后溶解,释放出残留 K +、Ni 2+和 Fe(CN) 6 3,在电极上作为结晶分解产物沉淀。这种副反应剥夺了活性材料 NiHCF 的电荷补偿 Fe,从而解释了观察到的容量衰减。











































 京公网安备 11010802027423号
京公网安备 11010802027423号